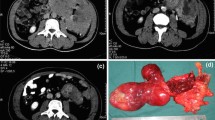
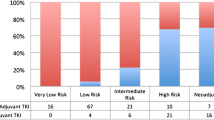
Abstract
Background
Neoadjuvant imatinib is used to downstage surgery for large and/or unfavorably located gastric gastrointestinal stromal tumors (GISTs), but data regarding minimally invasive surgery (MIS) after neoadjuvant imatinib are limited.
Patients and Methods
We analyzed patients undergoing resection of nonmetastatic primary gastric GISTs larger than or equal to 4.5 cm in diameter at our institution between 2009 and 2020, as no tumors below this size received neoadjuvant imatinib.
Results
We identified 71 patients, 43 of whom (61%) received neoadjuvant imatinib. Patients receiving neoadjuvant imatinib had larger tumors at diagnosis [median diameter 8.6 cm (range 4.5–25 cm) versus 5.9 cm (range 4.5–11 cm), p < 0.01]. After a median 7.2 months of imatinib, tumors shrank by a median 34% in diameter, such that there was no longer a significant size difference at time of surgery between groups (median 6.3 cm versus 5.9 cm, p = 0.69). Of 29 patients for whom neoadjuvant imatinib was used to facilitate MIS, 21 (72%) underwent successful MIS, which accounted for 49% of the entire neoadjuvant cohort. In a multivariable regression model, smaller tumor size at time of surgery was predictive of successful MIS, but tumor location was not.
Conclusions
Neoadjuvant imatinib caused significant tumor shrinkage, and MIS was successful in 72% of cases for which neoadjuvant imatinib was intended to facilitate it. Smaller tumor size at time of surgery, but not tumor location, was associated with successful MIS, which may help inform patient selection for neoadjuvant imatinib.


Similar content being viewed by others
References
Casali PG, Le Cesne A, Poveda Velasco A, et al. Time to definitive failure to the first tyrosine kinase inhibitor in localized GI stromal tumors treated with imatinib as an adjuvant: a European organisation for research and treatment of cancer soft tissue and bone sarcoma group intergroup randomized trial in collaboration with the Australasian gastro-intestinal trials group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J Clin Oncol. 2015;33:4276–83.
Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104.
Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265–72.
Cavnar MJ, Seier K, Gonen M, et al. Prognostic factors after neoadjuvant imatinib for newly diagnosed primary gastrointestinal stromal tumor. J Gastrointest Surg 2020.
Raut CP, Espat NJ, Maki RG, et al. Efficacy and tolerability of 5-year adjuvant imatinib treatment for patients with resected intermediate- or high-risk primary gastrointestinal stromal tumor: the PERSIST-5 clinical trial. JAMA Oncol. 2018;4:e184060.
Emory TS, Sobin LH, Lukes L, Lee DH, O’Leary TJ. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol. 1999;23:82–7.
Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100:162–8.
Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009;10:1045–52.
Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol. 2009;35:739–45.
Kurokawa Y, Yang HK, Cho H, et al. Phase II study of neoadjuvant imatinib in large gastrointestinal stromal tumours of the stomach. Br J Cancer. 2017;117:25–32.
Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol. 2013;20:2937–43. https://doi.org/10.1245/s10434-013-3013-7.
Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9.
Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–2.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1 1). Eur J Cancer. 2009;45:228–47.
Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive surgery for metastatic gastrointestinal stromal tumors treated with tyrosine kinase inhibitors: a 2-institutional analysis. Ann Surg. 2018;268:296–302.
Kawabata K, Takahashi T, Nakajima K, et al. Laparoscopic resection of a huge gastric gastrointestinal stromal tumor after neoadjuvant chemotherapy—a case report. Gan To Kagaku Ryoho. 2020;47:670–2.
Yoshioka S, Tazawa H, Saito A, et al. Laparoscopic resection for a large gastrointestinal stromal tumor (GIST) with diaphragm invasion following preoperative imatinib treatment: a case report. Int J Surg Case Rep. 2021;81:105727.
Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83.
Tirumani SH, Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH, Raut CP. Radiologic assessment of earliest, best, and plateau response of gastrointestinal stromal tumors to neoadjuvant imatinib prior to successful surgical resection. Eur J Surg Oncol. 2014;40:420–8.
Joo I, Lee JM, Lee ES, et al. Preoperative CT classification of the resectability of pancreatic cancer: interobserver agreement. Radiology. 2019;293:343–9.
Mohammad WM, Martel G, Mimeault R, Fairfull-Smith RJ, Auer RC, Balaa FK. Evaluating agreement regarding the resectability of colorectal liver metastases: a national case-based survey of hepatic surgeons. HPB (Oxford). 2012;14:291–7.
Balbona J, Chen L, Malafa MP, et al. Outcomes of gastric resection in the establishment of a comprehensive oncologic robotic program. J Surg Res. 2020;252:30–6.
Maggioni C, Shida A, Mancini R, Ioni L, Pernazza G. Safety profile and oncological outcomes of gastric gastrointestinal stromal tumors (GISTs) robotic resection: single center experience. Int J Med Robot. 2019;15:e2031.
Winder A, Strauss DC, Jones RL, et al. Robotic surgery for gastric gastrointestinal stromal tumors: a single center case series. J Surg Oncol. 2020. https://doi.org/10.1016/j.ejso.2019.11.454.
Efficace F, Baccarani M, Breccia M, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27:1511–9.
Poort H, van der Graaf WT, Tielen R, et al. Prevalence, impact, and correlates of severe fatigue in patients with gastrointestinal stromal tumors. J Pain Symptom Manage. 2016;52:265–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, G.Z., Fairweather, M., Raut, C.P. et al. Use of Neoadjuvant Imatinib to Facilitate Minimally Invasive Resection of Gastric Gastrointestinal Stromal Tumors. Ann Surg Oncol 29, 7104–7113 (2022). https://doi.org/10.1245/s10434-022-11891-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11891-9