Abstract
Background
The outcome of pancreatic ductal adenocarcinoma (PDAC) is unsatisfactory, and the identification of novel therapeutic targets is urgently needed. Clinical studies on the antisense oligonucleotide that targets clusterin (CLU) expression have been conducted and have shown efficacy in other cancers. We aimed to investigate the effects of CLU in PDAC and the underlying mechanisms with a view to the clinical application of existing drugs.
Methods
We knocked down CLU in PDAC cells and evaluated changes in cell proliferation. To elucidate the mechanism responsible for these changes, we performed western blot analysis, cell cycle assay, and senescence-associated β-galactosidase (SA-β-gal) staining. To evaluate the clinical significance of CLU, immunohistochemistry was performed, and CLU expression was analyzed in specimens resected from PDAC patients not treated with preoperative chemotherapy.
Results
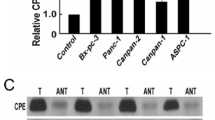
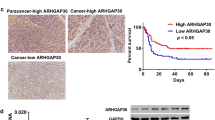
Knockdown of CLU significantly decreased cell proliferation and did not induce apoptosis, but did induce cellular senescence by increasing the percentage of G1-phase and SA-β-gal staining-positive cells. A marker of DNA damage such as γH2AX and factors related to cellular senescence, such as p21 and the senescence-associated secretory phenotype, were upregulated by knockdown of CLU. CLU expression in resected PDAC specimens was located in the cytoplasm of tumor cells and revealed significantly better recurrence-free survival and overall survival in the CLU-low group than in the CLU-high group.
Conclusions
We identified that CLU inhibition leads to cellular senescence in PDAC. Our findings suggest that CLU is a novel therapeutic target that contributes to the prognosis of PDAC by inducing cellular senescence.




Similar content being viewed by others
References
Barman S, Fatima I, Singh AB, Dhawan P. Pancreatic cancer and therapy: role and regulation of cancer stem cells. Int J Mol Sci. 2021;22(9):4765. https://doi.org/10.3390/ijms22094765.
Heredia-Soto V, Rodríguez-Salas N, Feliu J. Liquid biopsy in pancreatic cancer: Are we ready to apply it in the clinical practice? Cancers (Basel). 2021;13(8):1986. https://doi.org/10.3390/cancers13081986.
Gablo NA, Prochazka V, Kala Z, Slaby O, Kiss I. Cell-free microRNAs as non-invasive diagnostic and prognostic biomarkers in pancreatic cancer. Curr Genomics. 2019;20(8):569–80. https://doi.org/10.2174/1389202921666191217095017.
Zheng W, Yao M, Wu M, Yang J, Yao D, Wang L. Secretory clusterin promotes hepatocellular carcinoma progression by facilitating cancer stem cell properties via AKT/GSK-3β/β-catenin axis. J Transl Med. 2020;18(1):1–16. https://doi.org/10.1186/s12967-020-02262-7.
Wei L, Xue T, Wang J, et al. Roles of clusterin in progression, chemoresistance and metastasis of human ovarian cancer. Int J Cancer. 2009;125(4):791–806. https://doi.org/10.1002/ijc.24316.
Trougakos IP, Lourda M, Antonelou MH, et al. Intracellular clusterin inhibits mitochondrial apoptosis by suppressing p53-activating stress signals and stabilizing the cytosolic Ku70-bax protein complex. Clin Cancer Res. 2009;15(1):48–59. https://doi.org/10.1158/1078-0432.CCR-08-1805.
Deb M, Sengupta D, Rath SK, et al. Clusterin gene is predominantly regulated by histone modifications in human colon cancer and ectopic expression of the nuclear isoform induces cell death. Biochim Biophys Acta Mol Basis Dis. 2015;1852(8):1630–45. https://doi.org/10.1016/j.bbadis.2015.04.021.
Uddin MS, Kabir MT, Begum MM, Islam MS, Behl T, Ashraf MGM. Exploring the role of CLU in the pathogenesis of Alzheimer’s disease. Neurotox Res. 2020;60. doi:https://doi.org/10.1007/s12640-020-00271-4
Yao M, Fang M, Zheng W, Dong Z, Yao D. Role of secretory clusterin in hepatocarcinogenesis. Transl Gastroenterol Hepatol. 2018;3:3–7. https://doi.org/10.21037/tgh.2018.07.13.
Wang X, Xie J, Lu X, et al. Melittin inhibits tumor growth and decreases resistance to gemcitabine by downregulating cholesterol pathway gene CLU in pancreatic ductal adenocarcinoma. Cancer Lett. 2017;399:1–9. https://doi.org/10.1016/j.canlet.2017.04.012.
Xu M, Chen X, Han Y, Ma C, Ma L, Li S. Clusterin silencing sensitizes pancreatic cancer MIA-PaCa-2 cells to gmcitabine via regulation of NF-KB/BCL-2 signaling. Int J Clin Exp Med. 2015;8(8):12476–86.
Xiu P, Dong X, Dong X, et al. Secretory clusterin contributes to oxaliplatin resistance by activating Akt pathway in hepatocellular carcinoma. Cancer Sci. 2013;104(3):375–82. https://doi.org/10.1111/cas.12088.
Zhou J, Chen X, Gilvary DL, et al. HMGB1 induction of clusterin creates a chemoresistant niche in human prostate tumor cells. Nat Publ Gr. 2015. https://doi.org/10.1038/srep15085.
Peng M, Deng J, Zhou S, et al. The role of clusterin in cancer metastasis. Cancer Manag Res. 2019;11:2405–14. https://doi.org/10.2147/CMAR.S196273.
Lau SH, Sham JST, Xie D, et al. Clusterin plays an important role in hepatocellular carcinoma metastasis. Oncogene. 2006;25(8):1242–50. https://doi.org/10.1038/sj.onc.1209141.
Wang C, Jiang K, Kang X, et al. Tumor-derived secretory clusterin induces epithelial-mesenchymal transition and facilitates hepatocellular carcinoma metastasis. Int J Biochem Cell Biol. 2012;44(12):2308–20. https://doi.org/10.1016/j.biocel.2012.09.012.
Wang C, Jin G, Jin H, Wang N, Luo Q, Zhang Y. Clusterin facilitates metastasis by EIF3I/Akt/MMP13 signaling in hepatocellular carcinoma. Oncotarget. 2015;6(5):2903–16.
Xiu P, Dong XF, Li XP, Li J. Clusterin: Review of research progress and looking ahead to direction in hepatocellular carcinoma. World J Gastroenterol. 2015;21(27):8262–70. https://doi.org/10.3748/wjg.v21.i27.8262.
Laskin JJ, Nicholas G, Lee C, et al. Phase I/II trial of custirsen (OGX-011), an inhibitor of clusterin, in combination with a gemcitabine and platinum regimen in patients with previously untreated advanced non-small cell lung cancer. J Thorac Oncol. 2012;7(3):579–86. https://doi.org/10.1097/JTO.0b013e31823f459c.
Beer TM, Hotte SJ, Saad F, et al. Custirsen (OGX-011) combined with cabazitaxel and prednisone versus cabazitaxel and prednisone alone in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (AFFINITY): a randomised, open-label, international, ph. Lancet Oncol. 2017;18(11):1532–42. https://doi.org/10.1016/S1470-2045(17)30605-8.
Lamoureux F, Baud’huin M, Ory B, et al. Clusterin Inhibition Using OGX-011 Synergistically Enhances Zoledronic Acid Activity in Osteosarcoma. Vol 5.; 2014. www.impactjournals.com/oncotarget/.
Shinke G, Yamada D, Eguchi H, et al. Role of histone deacetylase 1 in distant metastasis of pancreatic ductal cancer. Cancer Sci. 2018;109(8):2520–31. https://doi.org/10.1111/cas.13700.
Iwagami Y, Eguchi H, Nagano H, et al. MiR-320c regulates gemcitabine-resistance in pancreatic cancer via SMARCC1. Br J Cancer. 2013;109(2):502–11. https://doi.org/10.1038/bjc.2013.320.
Mukai Y, Yamada D, Eguchi H, et al. Vitamin D supplementation is a promising therapy for pancreatic ductal adenocarcinoma in conjunction with current chemoradiation therapy. Ann Surg Oncol. 2018;25(7):1868–79. https://doi.org/10.1245/s10434-018-6431-8.
Jin J, Kim JM, Hur YS, et al. Clinical significance of clusterin expression in pancreatic adenocarcinoma. World J Surg Oncol. 2012;10(1):1. https://doi.org/10.1186/1477-7819-10-146.
Foster EM, Dangla-Valls A, Lovestone S, Ribe EM, Buckley NJ. Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies. Front Neurosci. 2019;13:1–27. https://doi.org/10.3389/fnins.2019.00164.
Rohne P, Prochnow H, Koch-Brandt C. The CLU-files: disentanglement of a mystery. Biomol Concepts. 2016;7(1):1–15. https://doi.org/10.1515/bmc-2015-0026.
Sánchez-Martín P, Komatsu M. Heparan sulfate and clusterin: cleaning squad for extracellular protein degradation. J Cell Biol. 2020;219(3):1–2. https://doi.org/10.1083/JCB.202001159.
Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7(9):909–15. https://doi.org/10.1038/ncb1291.
He J, Zhu G, Gao L, et al. Fra-1 is upregulated in gastric cancer tissues and affects the PI3K/Akt and p53 signaling pathway in gastric cancer. Int J Oncol. 2015;47(5):1725–34. https://doi.org/10.3892/ijo.2015.3146.
Zhong J, Yu X, Dong X, et al. Therapeutic role of meloxicam targeting secretory clusterin-mediated invasion in hepatocellular carcinoma cells. Oncol Lett. 2018;15(5):7191–9. https://doi.org/10.3892/ol.2018.8186.
Iwagami Y, Huang CK, Olsen MJ, et al. Aspartate β-hydroxylase modulates cellular senescence through glycogen synthase kinase 3β in hepatocellular carcinoma. Hepatology. 2016;63(4):1213–26. https://doi.org/10.1002/hep.28411.
Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. https://doi.org/10.1146/annurev-physiol-030212-183653.
Liu P, Tang Q, Chen M, et al. Hepatocellular senescence: immunosurveillance and future senescence-induced therapy in hepatocellular carcinoma. Front Oncol. 2020;10:1–14. https://doi.org/10.3389/fonc.2020.589908.
Moore PS, Sipos B, Orlandini S, et al. Genetic profile of 22 pancreatic carcinoma cell lines: analysis of K-ras, p53, p16 and DPC4/Smad4. Virchows Arch. 2001;439(6):798–802. https://doi.org/10.1007/s004280100474.
Hu S, Chen X, Xu X, et al. STRAP as a new therapeutic target for poor prognosis of pancreatic ductal adenocarcinoma patients mainly caused by TP53 mutation. Front Oncol. 2020;10:1–14. https://doi.org/10.3389/fonc.2020.594224.
Habiel DM, Camelo A, Espindola M, et al. Divergent roles for clusterin in lung injury and repair. Sci Rep. 2017;7(1):1–14. https://doi.org/10.1038/s41598-017-15670-5.
Mu L, Yang F, Guo D, Li P, Zhang M. Overexpression of secretory clusterin (sCLU) induces chemotherapy resistance in human gastric cancer cells by targeting miR-195-5p. Bioengineered. 2020;11(1):472–83. https://doi.org/10.1080/21655979.2020.1747825.
Wang Q, Cao W, Su Q, Liu Z, Zhang L. Clusterin silencing inhibits proliferation and reduces invasion in human laryngeal squamous carcinoma cells. World J Surg Oncol. 2014;12(1):1–8. https://doi.org/10.1186/1477-7819-12-124.
Sasaki N, Gomi F, Yoshimura H, Yamamoto M, Matsuda Y. Cancer cell proliferation and invasion while inducing senescence: evidence for senolytic therapy. 2020.
Iida-Norita R, Kawamura M, Suzuki Y, et al. Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma. Cancer Sci. 2019;110(7):2296–308. https://doi.org/10.1111/cas.14041.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no conflicts of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitsufuji, S., Iwagami, Y., Kobayashi, S. et al. Inhibition of Clusterin Represses Proliferation by Inducing Cellular Senescence in Pancreatic Cancer. Ann Surg Oncol 29, 4937–4946 (2022). https://doi.org/10.1245/s10434-022-11668-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11668-0