Abstract
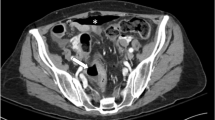
Bevacizumab is the first U.S. Food and Drug Association-approved vascular endothelial growth factor-targeted agent that greatly increases progression-free and overall survival in combination with standard chemotherapy regimens in patients with metastatic colorectal cancer. Although bevacizumab is generally well tolerated, some serious adverse events have occurred in some patients in clinical trials, including arterial thromboembolism and gastrointestinal (GI) perforation. GI perforation was first observed in the pivotal phase 3 trial, in which six events occurred in bevacizumab group (1.5%), compared with no events in the control group. Since then, similar rates of GI perforation have been observed in other large trials. Typical presentation was abdominal pain associated with constipation and vomiting. Such events occurred throughout treatment and were not correlated with duration of exposure. No difference in rate of GI perforations was found in patients who did and did not have a baseline history of peptic ulcer disease, diverticulosis, and history of chronic use of nonsteroidal anti-inflammatory drugs. However, the incidence of GI perforation seemed to be higher in patients with primary tumor intact, recent history of sigmoidoscopy or colonoscopy, or previous adjuvant radiotherapy, but it is necessary to confirm these preliminary findings by multivariate analyses. The mechanism responsible for causing GI perforation is not known and may be multifactorial. Bevacizumab should be permanently discontinued in patients who develop GI perforation. This article reviews the incidence, presentation, pathogenesis, risk factors, and management of GI perforation in patients with colorectal cancer who are treated with bevacizumab.
Similar content being viewed by others
REFERENCES
American Cancer Society. Colorectal cancer facts and figures—special edition 2005. Available at: http://www.cancer.org/docroot/STT/content/STT_1x_Colorectal_Cancer_Facts_and_Figures_-_Special_Edition_2005.asp. Accessed January 28, 2007
National Comprehensive Cancer Network. Clinical practice guidelines for oncology: Colon cancer version 2.2006. Available at: http://www.nccn.org/professionals/physician_gls/PDF/colon.pdf. Accessed June 18, 2006
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002; 20:4368–80
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285:1182–6
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407:249–57
Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2002; 2:727–39
Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993; 362:841–4
Emanuel S, Gruninger RH, Fuentes-Pesquera A, et al. A vascular endothelial growth factor receptor-2 kinase inhibitor potentiates the activity of the conventional chemotherapeutic agents paclitaxel and doxorubicin in tumor xenograft models. Mol Pharmacol 2004; 66:635–47
Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockade of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res 1999; 59:3374–8
Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3:391–400
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350:2335–42
Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol 2005; 23:3697–705
Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin/fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: final analysis of the TREE study (abstract 3510). Paper presented at American Society of Clinical Oncology 2006
Giantonio BJ, Catalano PJ, Meropol NJ, et al. High-dose bevacizumab improves survival when combined with FOLFOX4 in previously treated advanced colorectal cancer: results from the Eastern Cooperative Oncology Group (ECOG) study E3200 (abstract 2). Paper presented at American Society of Clinical Oncology 2005
Saltz LB, Lenz H, Kindler H, et al. Interim report of randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetuximab/bevacizumab (CB) in irinotecan-refractory colorectal cancer (abstract 169b). Paper presented at American Society of Clinical Oncology Gastrointestinal Symposium 2005
Hedrick E, Kozloff M, Hainsworth J, et al. Safety of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRiTE) (abstract 3536). Paper presented at American Society of Clinical Oncology 2006
Kozloff M, Hainsworth J, Badarinath S, et al. Efficacy of bevacizumab plus chemotherapy as first-line treatment of patients with metastatic colorectal cancer: updated results from a large observational registry in the US (BRiTE) (abstract 3537). Paper presented at American Society of Clinical Oncology 2006
Sugrue M, Kozloff M, Hainsworth J, et al. Risk factors for gastrointestinal perforations in patients with metastatic colorectal cancer receiving bevacizumab plus chemotherapy (abstract 3535). Paper presented at American Society of Clinical Oncology 2006
Kretzschmar A, Cunningham D, Berry S, et al. Incidence of gastrointestinal perforations and bleeding in patients starting bevacizumab treatment in first-line mCRC without primary tumour resection: preliminary results from the First BEATrial (abstract 248). Paper presented at American Society of Clinical Oncology Gastrointestinal Symposium 2006
Van Cutsem E, Michael M, Berry S, et al. Preliminary safety of bevacizumab with first-line FOLFOX, CAPOX, FOLFIRI and capecitabine for mCRC: First BEATrial (abstract 250). Paper presented at American Society of Clinical Oncology Gastrointestinal Symposium 2006
Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005; 23:3502–8
Kabbinavar F, Hurwitz H, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 2003; 21:60–5
Hamilton W, Round A, Sharp D, Peters TJ. Clinical features of colorectal cancer before diagnosis: a population-based case-control study. Br J Cancer 2005; 93:399–405
Gatto N, Frucht H, Sundararajan V, et al. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst 2003; 95:230–6
Beers MH, Berkow R, eds. Gastrointestinal disorders: acute abdomen and surgical gastroenterology. In: The Merck Manual of Diagnosis and Therapy. 17th ed. Whitehouse Station, NJ: Merck & Co., Inc., 1999; pp 269–275
Murray MJ, Gonze MD, Nowak LR, Cobb CF. Serum d(-)-lactate levels as an aid to diagnosing acute intestinal ischemia. Am J Surg 1994; 167:575–8
Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 2006; 290:H547–59
Betsholtz C, Armulik A. Homeostatic functions of vascular endothelial growth factor in adult microvasculature. Am J Physiol Heart Circ Physiol 2006; 290:H509–11
Kamba T, Tam BYY, Hashizume H, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 2006; 290:H560–76
Yang R, Thomas GR, Bunting S, et al. Effects of vascular endothelial growth factor on hemodynamics and cardiac performance. J Cardiovasc Pharmacol 1996; 27:838–44
Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology 2005; 69(Suppl 3):25–33
Genentech Inc. Avastin prescribing information. June 2006
Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol 2005; 91:173–80
Berry S, Michael M, Kretzschmar A, et al. Lack of effect of starting bevacizumab shortly after venous access device implantation on wound healing/bleeding complications: preliminary results from First BEAT (abstract 245). Paper presented at American Society of Clinical Oncology Gastrointestinal Symposium 2005
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saif, M.W., Elfiky, A. & Salem, R.R. Gastrointestinal Perforation Due to Bevacizumab in Colorectal Cancer. Ann Surg Oncol 14, 1860–1869 (2007). https://doi.org/10.1245/s10434-006-9337-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9337-9