Abstract
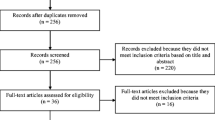
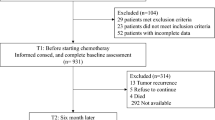
Health-related quality of life (HRQL) is a fundamental outcome in surgical oncology and culturally valid tools are essential for this purpose. Our aim was to validate the Mexican-Spanish versions of the European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life Questionnaire QLQ-C30 and the QLQ-STO22 disease-specific questionnaire module in Mexican patients with gastric cancer (GC). The translation procedure followed EORTC guidelines. Both instruments were completed by patients with GC and analyses were performed within three clinically distinct groups: (1) patients undergoing palliative treatment, (2) patients undergoing treatment with curative intent, and (3) GC survivors. Tests for reliability and validity were performed. One hundred and fifty patients (mean age 54.2 years) completed both questionnaires. Sixty-seven, 55, and 28 patients were allocated to groups 1, 2, and 3, respectively. Compliance rates were high, and questionnaires were well-accepted. Survivors of treatment for GC reported better functional HRQL scores and lower symptom scores than patients in group 2 who were currently undergoing treatment. Patients selected for potentially curative treatment had better HRQL than group 1 (palliative treatments). Scales in the QLQ-C30 and QLQ-STO22 distinguished between other clinically distinct groups of patients. Cronbach’s α coefficients of 14 scales of both questionnaires were >0.7. Multitrait scaling analysis demonstrated good convergent and discriminant validity. Test–retest scores were consistent. We conclude that the Mexican-Spanish versions of EORTC QLQ-C30 and QLQ-C22 questionnaires are reliable and valid for HRQL measurement in patients with GC and are therefore recommended for use in clinical trials of Mexican community.
Similar content being viewed by others
References
Ferlay J, Bray F, Pisani P, Parkin DM. Globocan 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5, version 2.0; IARC, Lyon, France; 2004.
Oñate-Ocaña LF. Gastric cancer in Mexico. Gastric Cancer. 2001;4:162–4.
Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39.
Blazeby JM, Avery K, Sprangers M, Pikhart H, Fayers P, Donovan J. Health-related quality of life measurement in randomized clinical trials in surgical oncology. J Clin Oncol. 2006;24:3178–86.
Fayers PM, Machin D, editors. Quality of life. The assessment, analysis, and interpretation of patient-reported outcomes. 2nd ed. West Sussex: Wiley; 2007.
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–9.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76.
Kemmler G, Holzner B, Kopp M, Dunser M, Margreiter R, Greil R, et al. Comparison of two quality-of-life instruments for cancer patients: the functional assessment of cancer therapy-general and the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-C30. J Clin Oncol. 1999;17:2932–40.
Functional Assessment of Chronic Illness Therapy (FACIT). http://www.facit.org/qview/qlist.aspx. Accessed 23 Oct 2008.
Blazeby JM, Conroy T, Bottomley A, et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO 22, to assess quality of life in patients with gastric cancer. Eur J Cancer. 2004;40:2260–8.
Conroy T, Marchal F, Blazeby JM. Quality of life in patients with oesophageal and gastric cancer: an overview. Oncology. 2006;70:391–402.
Huang CC, Lien HH, Sung YC, Liu HT, Chie WC. Quality of life of patients with gastric cancer in Taiwan: validation and clinical application of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQSTO22. Psychooncology. 2007;16:945–9.
Greene FL, Page DL, Fleming ID, Fritz A, Balch CM; American Joint Committee on Cancer. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002.
Koller M, Aaronson NK, Blazeby JM, et al. Translation procedures for standardised quality of life questionnaires: the European Organisation for Research and Treatment of Cancer (EORTC) approach. Eur J Cancer. 2007;43:1810–20.
Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. The EORTC QLQ-C30 scoring manual. 3rd ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001.
Tabachnik BJ, Fidell LS. Using multivariate statistics. London: Harper & Row; 1993.
Kaptein AA, Morita S, Sakamoto J. Quality of life in gastric cancer. World J Gastroenterol. 2005;11:3189–96.
Scott NW, Fayers PM, Aaronson NK, et al. The relationship between overall quality of life and its subdimensions was influenced by culture: analysis of an international database. J Clin Epidemiol. 2008;61:788–95.
Morita S, Kaptein AA, Oba K, Sakamoto J. The domain structure of the EORTC QLQ-STO22 supported by Japanese validation data. Psychooncology. 2008;17:474–9.
Silpakit C, Sirilerttrakul S, Jirajarus M, Sirisinha T, Sirachainan E, Ratanatharathorn V. The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): validation study of the Thai version. Qual Life Res. 2006;15:167–72.
Nourissat A, Vasson MP, Merrouche Y, et al. Relationship between nutritional status and quality of life in patients with cancer. Eur J Cancer. 2008;44:1238–42.
Park SE, Lee WK, Chung M, Bang SM, Cho EK, Lee JH, et al. Quality of life in patients with advanced gastric cancer treated with second-line chemotherapy. Cancer Chemother Pharmacol. 2006;57:289–94.
Acknowledgements
We thank the European Organization for Research and Treatment of Cancer Quality of Life Group for their extensive support in the translation and validation procedures of the C30 and STO22 questionnaires. We thank Maggie Brunner, M.A., for her English-language editorial review, as well as Alejandra García-Hubard and Blanca Rosas-Rosas because of their kind support in the logistics of this protocol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oñate-Ocaña, L.F., Alcántara-Pilar, A., Vilar-Compte, D. et al. Validation of the Mexican Spanish Version of the EORTC C30 and STO22 Questionnaires for the Evaluation of Health-Related Quality of Life in Patients with Gastric Cancer. Ann Surg Oncol 16, 88–95 (2009). https://doi.org/10.1245/s10434-008-0175-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-008-0175-9