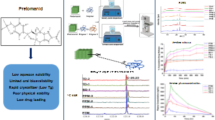
Abstract
Solid dispersion (SD) technology is one of the most widely preferred solubility enhancement methods, especially for Biopharmaceutics classification system class II and IV drugs. Since the last decade, its application for the dual purpose of solubility hike and modified release using novel carriers has been in demand for its added advantages. Spray drying is a commercially accepted technique with high aspects of scalability and product characteristics. The current study used spray-dried dispersion to design delayed release capsule for the proton pump inhibitor esomeprazole. The SD carrier hydroxypropyl methylcellulose acetate succinate-medium grade (HPMCAS-MF) enhanced solubility, inhibited precipitation of saturated drug solutions, and allowed enteric release owing to its solubility above pH 6. The proposed approach avoided compression, coating with enteric polymers, and the development of multi-particulate pellet-based formulations, improving manufacturing feasibility. The formulation was optimized using Box-Behnken design, considering significant formulation variables like HPMCAS-MF proportion and critical process parameters like feed flow rate and inlet temperature. The optimized spray-dried dispersion were characterized based on Fourier transform infrared spectroscopy (FTIR), differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and scanning electron microscopy (SEM) and also evaluated for solubility, in vitro drug release, residual solvent content, and stability testing. Response surface methodology optimization anticipated that formulation variables affected solubility and release profile, whereas CPPs affected yield. The design space was developed via overlay plot based on constraints specified to attain the desired response and validated using three checkpoint batches with desirability 1. FTIR showed active pharmaceutical ingredient-polymer compatibility. Particle size and SEM studies showed spherical particles with an average Z-value of 1.8 µ. DSC and PXRD confirmed SD’s amorphous nature. The drug release investigation and release kinetics prediction utilizing DD-solver software showed a 2-h lag time with > 90% cumulative drug release up to 4 h for the DR formulation.
Graphical abstract
ESM SDD were prepared by spray drying technique using the novel solid dispersion carrier HPMCAS-MF to serve the dual purpose of solubility enhancement and delayed release. The ratio of API:carrier and process variables like feed flow rate and inlet temperature were varied using the Box-Behnken Design to determine the design space of optimized product to procure the desired characteristics of solubility improvement compared to crystalline API and delayed release of PPI to avoid the degradation in the gastric environment. The developed formulation represents several benefits over the already existing marketed products.








Similar content being viewed by others
Data Availability
Data availability statement is not applicable.
References
Kwon TK, Kang JH, Na SB, Kim JH, Kim YI, Kim DW, et al. novel esomeprazole magnesium-loaded dual-release mini-tablet polycap: formulation, optimization, characterization, and in vivo evaluation in beagle dogs. Pharmaceutics. 2022;14:1411.
Esomeprazole drugbank [Internet]. [Cited 2022 Nov 23]. Available from: https://go.drugbank.com/salts/DBSALT001222. Accessed 10 Feb 2023.
Kan SL, Lu J, Liu JP, Zhao Y. Preparation and in vitro/in vivo evaluation of esomeprazole magnesium-modified release pellets. Drug Deliv. 2016;23:866–73.
Kanwar K, Gautam SP. Qualitative portrayal of esomeprazole magnesium by exploring diverse analytical and investigative approaches. J Pharm Res Int. 2021;33(34B):52–68.
Nexium rxlist. https://www.rxlist.com/nexium-drug.htm#description.
Srebro J, Brniak W, Mendyk A. Formulation of dosage forms with proton pump inhibitors: state of the art, challenges and future perspectives. Pharmaceutics. 2022;14:2043.
Pradhan R, Tran TH, Kim SY, Woo KB, Choi YJ, Choi HG, et al. Preparation and characterization of fast dissolving flurbiprofen and esomeprazole solid dispersion using spray drying technique. Int J Pharm. 2016;502:38–46.
Van Nguyen H, Baek N, Lee BJ. Enhanced gastric stability of esomeprazole by molecular interaction and modulation of microenvironmental pH with alkalizers in solid dispersion. Int J Pharm. 2017;523:189–202.
Xie Y, Xie P, Song X, Tang X, Song H. Preparation of esomeprazole zinc solid dispersion and study on its pharmacokinetics. Int J Pharm. 2008;360:53–7.
Beneš M, Pekárek T, Beránek J, Havlíček J, Krejčík L, Šimek M, et al. Methods for the preparation of amorphous solid dispersions – a comparative study. J Drug Deliv Sci Technol [Internet]. 2017;38:125–34. https://doi.org/10.1016/j.jddst.2017.02.005.
Patel K, Shah S, Patel J. Solid dispersion technology as a formulation strategy for the fabrication of modified release dosage forms: a comprehensive review. DARU, J Pharm Sci. 2022;30:165–89.
Malkawi R, Malkawi WI, Al-Mahmoud Y, Tawalbeh J. Current trends on solid dispersions: past, present, and future. Adv Pharmacol Pharm Sci. 2022.
Raina SA, Zhang GGZ, Alonzo DE, Wu J, Zhu D, Catron ND, et al. Enhancements and limits in drug membrane transport using supersaturated solutions of poorly water soluble drugs. J Pharm Sci. 2014;103:2736–48.
Bhujbal SV, Mitra B, Jain U, Gong Y, Agrawal A, Karki S, et al. Pharmaceutical amorphous solid dispersion: a review of manufacturing strategies. Acta Pharm Sinica B. 2021;11:2505–36. https://doi.org/10.1016/j.apsb.2021.05.014.
De Mohac LM, Caruana R, Pavia FC, Cavallaro G, Giammona G, Licciardi M. Multicomponent solid dispersion as a formulation strategy to improve drug permeation: a case study on the anti-colorectal cancer irinotecan. J Drug Deliv Sci Technol. 2019;52:346–54.
Chen Y, Shi Q, Chen Z, Zheng J, Xu H, Li J, et al. Preparation and characterization of emulsified solid dispersions containing docetaxel. Arch Pharmacal Res. 2011;34:1909–17.
Ke P, Qi S, Sadowski G, Ouyang D. Solid dispersion - a pragmatic method to improve the bioavailability of poorly soluble drugs. Computational Pharmaceutics: Application of Molecular Modeling in Drug Delivery. 2015;81–100.
Pandi P, Bulusu R, Kommineni N, Khan W, Singh M. Amorphous solid dispersions: an update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int J Pharm [Internet]. 2020;586:119560. https://doi.org/10.1016/j.ijpharm.2020.119560.
DeBoyace K, Wildfong PLD. The application of modeling and prediction to the formation and stability of amorphous solid dispersions. J Pharm Sci [Internet]. 2018;107:57–74. https://doi.org/10.1016/j.xphs.2017.03.029.
Sahoo A, Kumar NSK, Suryanarayanan R. Crosslinking: an avenue to develop stable amorphous solid dispersion with high drug loading and tailored physical stability. J Control Release [Internet]. 2019;311–312:212–24. https://doi.org/10.1016/j.jconrel.2019.09.007.
He Y, Ho C. Amorphous solid dispersions: utilization and challenges in drug discovery and development. J Pharm Sci [Internet]. 2015;104:3237–58. https://doi.org/10.1002/jps.24541.
Tekade AR, Yadav JN. A review on solid dispersion and carriers used therein for solubility enhancement of poorly water soluble drugs. Adv Pharm Bull [Internet]. 2020;10:359–69. https://doi.org/10.34172/apb.2020.044.
Nair AR, Lakshman YD, Anand VSK, Sree KSN, Bhat K, Dengale SJ. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS Pharm Sci Tech. 2020;21:1–20.
Sharma KS, Sahoo J, Agrawal S, Kumari A. Solid dispersions: a technology for improving bioavailability. J Anal Pharm Res. 2019;8:127–33.
Ulmanu M, Anger I. Physical and chemical properties. Handbook of Natural Zeolites. 2012;70–102.
Deng Y, Liang Q, Wang Y, Zhang X, Yan C, He Y. The inhibiting role of hydroxypropylmethylcellulose acetate succinate on piperine crystallization to enhance its dissolution from its amorphous solid dispersion and permeability. RSC Adv. 2019;9:39523–31.
Butreddy A. Hydroxypropyl methylcellulose acetate succinate as an exceptional polymer for amorphous solid dispersion formulations: a review from bench to clinic. Eur J Pharm Biopharm. 2022.
Pinto JMO, Leão AF, Riekes MK, França MT, Stulzer HK. HPMCAS as an effective precipitation inhibitor in amorphous solid dispersions of the poorly soluble drug candesartan cilexetil. Carbohyd Polym. 2018;184:199–206.
Sarabu S, Kallakunta VR, Bandari S, Batra A, Bi V, Durig T, et al. Hypromellose acetate succinate based amorphous solid dispersions via hot melt extrusion: effect of drug physicochemical properties. Carbohyd Polym. 2020;233: 115828.
Tanno F, Nishiyama Y, Kokubo H, Obara S. Evaluation of hypromellose acetate succinate (HPMCAS) as a carrier in solid dispersions. Drug Dev Ind Pharm. 2004;30:9–17.
Mahmah O, Tabbakh R, Kelly A, Paradkar A. A comparative study of the effect of spray drying and hot-melt extrusion on the properties of amorphous solid dispersions containing felodipine. J Pharm Pharmacol. 2014;66:275–84.
Vasconcelos T, Marques S, das Neves J, Sarmento B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv Drug Deliv Rev. 2016;100:85–101.
Slámová M, Školáková T, Školáková A, Patera J, Zámostný P. Preparation of solid dispersions with respect to the dissolution rate of active substance. J Drug Deliv Sci Technol. 2020;56:101518. https://doi.org/10.1016/j.jddst.2020.101518.
Tran P, Pyo YC, Kim DH, Lee SE, Kim JK, Park JS. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharm. 2019;11:1–26.
Broadbent A, Bennette N. Fundamentals of spray-dried dispersion technology. A SpeciAl Advertising Section. 2015.
Szabó E, Záhonyi P, Brecska D, Galata DL, Mészáros LA, Madarász L, et al. Comparison of amorphous solid dispersions of spironolactone prepared by spray drying and electrospinning: the influence of the preparation method on the dissolution properties. Mol Pharm. 2021;18:317–27.
Poudel S, Kim DW. Developing ph-modulated spray dried amorphous solid dispersion of candesartan cilexetil with enhanced in vitro and in vivo performance. Pharmaceutics. 2021;13:497.
Surti N, Mahajan AN, Patel D, Patel A, Surti Z. Spray dried solid dispersion of repaglinide using hypromellose acetate succinate: in vitro and in vivo characterization. Drug Dev Ind Pharm. 2020;46:1622–31.
Mustafa WW, Fletcher J, Khoder M, Alany RG. Solid dispersions of gefitinib prepared by spray drying with improved mucoadhesive and drug dissolution properties. AAPS Pharm Sci Tech. 2022;23:41.
Li Y, Mann AKP, Zhang D, Yang Z. Processing impact on in vitro and in vivo performance of solid dispersions—a comparison between hot-melt extrusion and spray drying. Pharmaceutics. 2021;13:1307.
Pohlen M, Lavrič Z, Prestidge C, Dreu R. Preparation, physicochemical characterisation and DoE optimisation of a spray-dried dry emulsion platform for delivery of a poorly soluble drug, simvastatin. AAPS Pharm Sci Tech. 2020;21:1–19.
Ajiboye AL, Nandi U, Galli M, Trivedi V. Olanzapine loaded nanostructured lipid carriers via high shear homogenization and ultrasonication. Sci Pharm. 2021;89:25.
Qushawy M, Nasr A, Swidan S, Mortagi Y. Development and characterization of glimepiride novel solid nanodispersion for improving its oral bioavailability. Sci Pharm. 2020;88:1–17.
Szabó E, Galata DL, Vass P, Hirsch E, Csontos I, Marosi G, et al. Continuous formulation approaches of amorphous solid dispersions: Significance of powder flow properties and feeding performance. Pharmaceutics. 2019;11:654.
Abdelhaleem Ali AM, Khames A, Alrobaian MM, Hamaidi M, Abourehab MAS. Glucosamine-paracetamol spray-dried solid dispersions with maximized intrinsic dissolution rate, bioavailability and decreased levels of in vivo toxic metabolites. Drug Des Dev Ther. 2018;12:3071–84.
Kaur P, Singh SK, Garg V, Gulati M, Vaidya Y. Optimization of spray drying process for formulation of solid dispersion containing polypeptide-k powder through quality by design approach. Powder Technol. 2015;284:1–11.
Muqtader Ahmed M, Fatima F, AbulKalam M, Alshamsan A, Soliman GA, Shaikh AA, et al. Development of spray-dried amorphous solid dispersions of tadalafil using glycyrrhizin for enhanced dissolution and aphrodisiac activity in male rats. Saudi Pharm J. 2020;28:1817–26.
Pontip B, Suchada P, Sriamornsak P. Effect of formulations and spray drying process conditions on physical properties of resveratrol spray-dried emulsions. Key engineering materials. Trans Tech Publications Ltd. 2019;819:246–51.
De PK, Sahana B, Rakshit S. Enhancement of dissolution rate and stability study of ofloxacin solid dispersion. Available from: www.pelagiaresearchlibrary.com. Accessed 10 Feb 2023
Ich. ICH guideline Q3C (R5) on impurities: guideline for residual solvents Part II and part III (PDE for Tetrahydrofuran and N-Methylpyrrolidone) End of consultation (deadline for comments) [Internet]. 2011. Available from: www.ema.europa.eu. Accessed 10 Feb 2023
Tashan E, Karakucuk A, Celebi N. Development of nanocrystal ziprasidone orally disintegrating tablets: optimization by using design of experiment and in vitro evaluation. AAPS Pharm Sci Tech. 2020;21:1–12.
Dong Q, Zhu J, Sui Q, Tang C, Wang X, Yu Y. Optimization of mobile phase for the determination of esomeprazole and related compounds and investigation of stress degradation by LC-MS. J Sep Sci. 2013;36:1200–8.
Acknowledgements
The authors KP, SS, and JP are thankful to L. J. Institute of Pharmacy, L J University, Ahmedabad, India, for providing necessary facilities and financial assistance to carry out the work, which is a part of Doctor of Philosophy (Ph.D.) research work of Ms. Kaushika Patel, to be submitted to Gujarat Technological University, Ahmedabad, India.
Author information
Authors and Affiliations
Contributions
The first author Kaushika Patel performed literature search, experimental work, study design and optimization, data analysis, data interpretation, writing; co-author Jaymin Patel contributed in figures, data analysis, data interpretation, editing; and corresponding author Shreeraj Shah provided supervision and critical review of the initial draft.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, K., Patel, J. & Shah, S. Development of Delayed Release Oral Formulation Comprising Esomeprazole Spray Dried Dispersion Utilizing Design of Experiment As An Optimization Strategy. AAPS PharmSciTech 24, 186 (2023). https://doi.org/10.1208/s12249-023-02642-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-023-02642-4