Abstract
Breast cancer metastasis is an important cause of death in patients with breast cancer and is closely related to circulating tumor cells (CTCs) and the metastatic microenvironment. As the most infiltrating immune cells in the tumor microenvironment (TME), tumor-associated macrophages (TAMs), which highly express sialic acid (SA) receptor (Siglec-1), are closely linked to tumor progression and metastasis. Furthermore, the surface of CTCs also highly expressed receptor (Selectin) for SA. A targeting ligand (SA-CH), composed of SA and cholesterol, was synthesized and modified on the surface of epirubicin (EPI)-loaded liposomes (EPI-SL) as an effective targeting delivery system. Liposomes were evaluated for characteristics, stability, in vitro release, cytotoxicity, cellular uptake, pharmacokinetics, tumor targeting, and pharmacodynamics. In vivo and in vitro experiments showed that EPI-SL enhanced EPI uptake by TAMs. In addition, cellular experiments showed that EPI-SL could also enhance the uptake of EPI by 4T1 cells, resulting in cytotoxicity second only to that of EPI solution. Pharmacodynamic experiments have shown that EPI-SL has optimal tumor inhibition with minimal toxicity, which can be ascribed to the fact that EPI-SL can deliver drugs to tumor based on TAMs and regulate TME through the depletion of TAMs. Our study demonstrated the significant potential of SA-modified liposomes in antitumor metastasis.
Graphical abstract
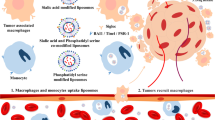
Schematic diagram of the role of SA-CH modified EPI-loaded liposomes in the model of breast cancer metastasis.









Similar content being viewed by others
Abbreviations
- TAMs:
-
Tumor-associated macrophages
- CTCs:
-
Circulating tumor cells
- EPI-S:
-
EPI solution
- Glu:
-
Glucose
- EPI-CL:
-
EPI-loaded conventional liposomes
- EPI-PL:
-
EPI-loaded PEGylated liposomes
- EPI-SL:
-
EPI-loaded SA-CH modified liposomes
- DiR-CL:
-
DiR-loaded conventional liposomes
- DiR-PL:
-
DiR-loaded PEGylated liposomes
- DiR-SL:
-
DiR-loaded SA-CH modified liposomes
References
Neophytou C, Boutsikos P, Papageorgis P. Molecular mechanisms and emerging therapeutic targets of triple-negative breast cancer metastasis. Front Oncol. 2018;8:31.
Stuart EC, Jarvis RM, Rosengren RJ. In vitro mechanism of action for the cytotoxicity elicited by the combination of epigallocatechin gallate and raloxifene in MDA-MB-231 cells. Oncol Rep. 2010;24:779–85.
Newman L. Breast cancer screening in low and middle-income countries. Best Pract Res Clin Obstet Gynaecol. 2022;83:15–23.
Hutchinson L. Breast cancer: challenges, controversies, breakthroughs. Nat Rev Clin Oncol. 2010;7:669–70.
Hong J, Shen Y-A, Hsu C-Y, et al. Targeting glutamine metabolism enhances responses to platinum-based chemotherapy in triple-negative breast cancers (TNBC). Genes Dis. 2022.
Al-Khanbashi M, Caramuta S, Alajmi AM, et al. Tissue and serum miRNA profile in locally advanced breast cancer (LABC) in response to neo-adjuvant chemotherapy (NAC) treatment. PLoS ONE. 2016;11: e0152032.
Ridwan SM, Hainfeld JF, Ross V, et al. Novel Iodine nanoparticles target vascular mimicry in intracerebral triple negative human MDA-MB-231 breast tumors. Sci Rep. 2021;11:1203.
Harquail J, Benzina S, Robichaud GA. MicroRNAs and breast cancer malignancy: an overview of miRNA-regulated cancer processes leading to metastasis. Cancer Biomark. 2012;11:269–80.
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92.
Kang Y, Pantel K. Tumor cell dissemination: emerging biological insights from animal models and cancer patients. Cancer Cell. 2013;23:573–81.
Lu Y, Lian S, Cheng Y, et al. Circulation patterns and seed-soil compatibility factors cooperate to cause cancer organ-specific metastasis. Exp Cell Res. 2019;375:62–72.
Zhou M, Zuo Q, Huang Y, Li L. Immunogenic hydrogel toolkit disturbing residual tumor “seeds” and pre-metastatic “soil” for inhibition of postoperative tumor recurrence and metastasis. Acta Pharm Sin B. 2022;12:3383–97.
Follain G, Herrmann D, Harlepp S, et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer. 2020;20:107–24.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–91.
Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–9.
Patil P, Madhuprasad M, Kumeria T, et al. Isolation of circulating tumour cells by physical means in a microfluidic device: a review. RSC Adv. 2015;5:89745–62.
Medeiros B, Allan AL. Molecular mechanisms of breast cancer metastasis to the lung: clinical and experimental perspectives. Int J Mol Sci. 2019;20:2272.
Yu X, Li B. Seed or soil: tracing the immune subsets in metastatic tumors. Cancer Cell. 2022;40:353–5.
Li R, Qi Y, Han M, et al. Computed tomography reveals microenvironment changes in premetastatic lung. Eur Radiol. 2021;31:4340–9.
Celia-Terrassa T, Kang Y. Metastatic niche functions and therapeutic opportunities. Nat Cell Biol. 2018;20:868–77.
Altorki NK, Markowitz GJ, Gao D, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer. 2019;19:9–31.
Fu L-Q, Du W-L, Cai M-H, et al. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353: 104119.
Hagemann T, Wilson J, Kulbe H, et al. Macrophages induce invasiveness of epithelial cancer cells via NF-κB and JNK. J Immunol. 2005;175:1197–205.
Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73.
Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51.
Luo Z, Wu S, Zhou J, et al. All-stage targeted therapy for the brain metastasis from triple-negative breast cancer. Acta Pharm Sin B. 2022.
Howard J, Goh CY, Gorzel KW, et al. The potential role of cofilin-1 in promoting triple negative breast cancer (TNBC) metastasis via the extracellular vesicles (EVs). Transl Oncol. 2022;15:101247.
Malla R, Puvalachetty K, Vempati RK, et al. Cancer stem cells and circulatory tumor cells promote breast cancer metastasis. Clin Breast Cancer. 2022;22:507–14.
Atukorale PU, Raghunathan SP, Raguveer V, et al. Nanoparticle encapsulation of synergistic immune agonists enables systemic co-delivery to tumor sites and interferon β-driven anti-tumor immunity. Cancer Res. 2019;79:5394–406.
Li Y, Qian D, Lin HP, et al. Nanoparticle-delivered miriplatin ultrasmall dots suppress triple negative breast cancer lung metastasis by targeting circulating tumor cells. J Control Release. 2021;329:833–46.
Choi MR, Stanton-Maxey KJ, Stanley JK, et al. A cellular Trojan horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–65.
Fu JJ, Wang D, Mei D, et al. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J Control Release. 2015;204:11–9.
Zhou S, Zhang T, Peng B, et al. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. Int J Pharm. 2017;523:203–16.
Ding J, Zhao D, Hu Y, et al. Terminating the renewal of tumor-associated macrophages: a sialic acid-based targeted delivery strategy for cancer immunotherapy. Int J Pharm. 2019;571: 118706.
Zhou S, Zhang T, Peng B, et al. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. Int J Pharm. 2017;523:203–16.
Zhang T, Zhou S, Hu L, et al. Polysialic acid-modifying liposomes for efficient delivery of epirubicin, in-vitro characterization and in-vivo evaluation. Int J Pharm. 2016;515:449–59.
Yang T, Cui FD, Choi MK, et al. Enhanced solubility and stability of PEGylated liposomal paclitaxel: in vitro and in vivo evaluation. Int J Pharm. 2007;338:317–26.
Matsuoka Y, Onohara E, Kojima N, Kuroda Y. Importance of particle size of oligomannose-coated liposomes for induction of Th1 immunity. Int Immunopharmacol. 2021;99: 108068.
Ikonen M, Murtomaki L, Kontturi K. Microcalorimetric and zeta potential study on binding of drugs on liposomes. Colloids Surf B Biointerfaces. 2010;78:275–82.
Duggan ST, Keating GMJD. Pegylated liposomal doxorubicin: a review of its use in metastatic breast cancer, ovarian cancer, multiple myeloma and AIDS-related Kaposi’s sarcoma. Drugs. 2011;71:2531–58.
Grabowska J, Lopez-Venegas MA, Affandi AJ, den Haan JMM. CD169(+) Macrophages capture and dendritic cells instruct: the interplay of the gatekeeper and the general of the immune system. Front Immunol. 2018;9:2472.
Nath D, Hartnell A, Happerfield L, et al. Macrophage–tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology. 1999;98:213.
Jayant S, Khandare JJ, Wang Y, et al. Targeted sialic acid-doxorubicin prodrugs for intracellular delivery and cancer treatment. Pharm Res. 2007;24:2120–30.
Steiniger B, Barth P, Herbst B, et al. The species-specific structure of microanatomical compartments in the human spleen: strongly sialoadhesin-positive macrophages occur in the perifollicular zone, but not in the marginal zone. Immulogy. 1997;92:307–16.
Florence AT. Nanoparticle flow: implications for drug delivery. 2006. p. 9–27.
Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10.
Yang VC. Personal perspectives and concerns over the so-called nanomedicine. J Control Release. 2019;311:322–3.
Widjaya AS, Liu Y, Yang Y, et al. Tumor-permeable smart liposomes by modulating the tumor microenvironment to improve the chemotherapy. J Control Release. 2022;344:62–79.
Catania G, Malaguti P, Gasparro S, et al. Activity of eribulin mesylate in brain metastasis from breast cancer: a stone in a pond? Oncology. 2018;94(Suppl 1):29–33.
Delozier T, Vernhes JC. Comparative study of adriamycin, epirubicin and mitoxantrone in cancer of the breast. Review of the literature. Bull Cancer. 1991;78:1013–25.
Song Y, Huang Z, Luo X, et al. Pharmacodynamics of liposomes modified with different chain length of sialic acid derivatives. Yao Xue Xue Bao. 2016;51:316–24.
Komohara Y, Takeya M. CAFs and TAMs: maestros of the tumour microenvironment. J Pathol. 2017;241:313–5.
Allavena P, Mantovani A. Immunology in the clinic review series; focus on cancer: tumour-associated macrophages: undisputed stars of the inflammatory tumour microenvironment. Clin Exp Immunol. 2012;167:195–205.
Lo UG, Pong RC, Yang D, et al. IFNgamma-induced IFIT5 promotes epithelial-to-mesenchymal transition in prostate cancer via miRNA processing. Cancer Res. 2019;79:1098–112.
Chen HC, Chou AS, Liu YC, et al. Induction of metastatic cancer stem cells from the NK/LAK-resistant floating, but not adherent, subset of the UP-LN1 carcinoma cell line by IFN-gamma. Lab Invest. 2011;91:1502–13.
Mumm JB, Emmerich J, Zhang X, et al. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer Cell. 2011;20:781–96.
Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliver Rev. 2012;64:206–12.
Sun TM, Zhang YS, Pang B, et al. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Edit. 2014;53:12320–64.
Alasvand N, Urbanska AM, Rahmati M, et al. Therapeutic nanoparticles for targeted delivery of anticancer drugs. In: Multifunctional systems for combined delivery, biosensing and diagnostics. 2017. p. 245–59.
Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–64.
Zhang L, Song XW, Qi QT, Liu WL. Interaction of DPPC liposomes with cholesterol and food protein during in vitro digestion using dynamic light scattering and FTIR spectroscopy analysis. Food Chem. 2022;375:131893.
von Filseck JM, Vanni S, Mesmin B, et al. A phosphatidylinositol-4-phosphate powered exchange mechanism to create a lipid gradient between membranes. Nat Commun. 2015;6:6671.
Arriaga LR, López-Montero I, Monroy F, et al. Stiffening effect of cholesterol on disordered lipid phases: a combined neutron spin echo + dynamic light scattering analysis of the bending elasticity of large unilamellar vesicles. Biophys J. 2009;96:3629–37.
Zhao L, Temelli F, Curtis JM, Chen LJFRI. Preparation of liposomes using supercritical carbon dioxide technology: effects of phospholipids and sterols. Food Res Int. 2015;77:63–72.
Kocisova E, Antalik A, Prochazka M. Drop coating deposition Raman spectroscopy of liposomes: role of cholesterol. Chem Phys Lipids. 2013;172–173:1–5.
Luo X. Pharmacodynamics of liposomes modified by sialic acid derivatives with different chain length.
Ercole F, Whittaker MR, Quinn JF, Davis TP. Cholesterol modified self-assemblies and their application to nanomedicine. Biomacromol. 2015;16:1886–914.
Funding
This study was supported by the Career Development Support Plan for Young and Middle-aged Teachers at the Shenyang Pharmaceutical University (ZDN2021009).
Author information
Authors and Affiliations
Contributions
Conceptualization: Xianmin Meng and Yanzhi Song; data curation: Xianmin Meng, Yihui Deng and Yanzhi Song; acquisition: Mingqi Wang, Kaituo Zhang, Zihan Xu and Tiantian Guo; writing: Xianmin Meng; analysis: Dezhi Sui and Meng Chen; supervision: Xinrong Liu. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, X., Wang, M., Zhang, K. et al. An Application of Tumor-Associated Macrophages as Immunotherapy Targets: Sialic Acid–Modified EPI-Loaded Liposomes Inhibit Breast Cancer Metastasis. AAPS PharmSciTech 23, 285 (2022). https://doi.org/10.1208/s12249-022-02432-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-022-02432-4