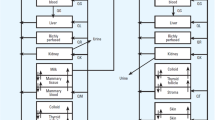
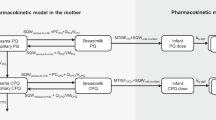
Abstract
Current methods to assess risk in infants exposed to maternal medication through breast milk do not specifically account for infants most vulnerable to high drug exposure. A workflow applied to lamotrigine incorporated variability in infant anatomy and physiology, milk intake volume, and milk concentration to predict infant exposure. An adult physiologically based pharmacokinetic model of lamotrigine was developed and evaluated. The model was scaled to account for growth and maturation of a virtual infant population (n=100). Daily infant doses were simulated using milk intake volume and concentration models described by a nonlinear equation of weight-normalized intake across infant age and a linear function on the relationship of observed milk concentrations and maternal doses, respectively. Average infant plasma concentration at steady state was obtained through simulation. Models were evaluated by comparing observed to simulated infant plasma concentrations from breastfeeding infants based on a 90% prediction interval (PI). Upper AUC ratio (UAR) was defined as a novel risk metric. Twenty-five paired (milk concentrations measured) and 18 unpaired (milk concentrations unknown) infant plasma samples were retrieved from the literature. Forty-four percent and 11% of the paired and unpaired infant plasma concentrations were outside of the 90% PI, respectively. Over all ages (0–7 months), unpaired predictions captured more observed infant plasma concentrations within 90% PI than paired. UAR was 0.18–0.44 when mothers received 200 mg lamotrigine, suggesting that infants can receive 18–44% of the exposure per dose as compared to adults. UARs determined for further medications could reveal trends to better classify at-risk mother-infant pairs.



Similar content being viewed by others
Change history
10 June 2021
A Correction to this paper has been published: https://doi.org/10.1208/s12248-021-00614-9
13 July 2021
A Correction to this paper has been published: https://doi.org/10.1208/s12248-021-00615-8
References
Food and Drug Administration. Clinical lactation studies: Considerations for study design, guidance for industry. 05/01/19.
Anderson PO, Sauberan JB. Modeling drug passage into human milk. Clin Pharmacol Ther. 2016;100(1):42–52.
Neville MC, Keller R, Seacat J, Lutes V, Neifert M, Casey C, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48(6):1375–86.
Maharaj AR, Edginton AN. Physiologically based pharmacokinetic modeling and simulation in pediatric drug development. CPT Pharmacometrics Syst Pharmacol. 2014;3(11):e150–e.
Cibert M, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model to predict neonate exposure to drugs during breast-feeding: application to lamotrigine. Fundam Clin Pharmacol. 2010;24(Suppl. 1):51.
Guedat MG, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model for predicting neonate dose after breast-feeding by women treated with clonidine. Int J Clin Pharm. 2011;33(2):318.
Rambeck B, Kurlemann G, Stodieck SR, May TW, Jürgens U. Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol. 1997;51(6):481–4.
Garessus EDG, Mielke H, Gundert-Remy U. Exposure of infants to isoniazid via breast milk after maternal drug intake of recommended doses is clinically insignificant irrespective of metaboliser status. A physiologically-based pharmacokinetic (PBPK) modelling approach to estimate drug exposure of infants via breast-feeding. Frontiers in pharmacology. 2019;10:5.
Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther. 2009;86(6):634–43.
Olagunju A, Rajoli R, Atoyebi S, Khoo S, Owen A, Siccardi M. Physiologically-based pharmacokinetic modelling of infant exposure to efavirenz through breastfeeding [version 1; peer review: 2 approved with reservations]. AAS Open Research. 2018;1(16).
Delaney SR, Malik PRV, Stefan C, Edginton AN, Colantonio DA, Ito S. Predicting escitalopram exposure to breastfeeding infants: Integrating analytical and in silico techniques. Clin Pharmacokinet. 2018;57(12):1603–11.
Kent JC, Mitoulas LR, Cregan MD, Ramsay DT. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3):e387-ee95.
Yeung CHT, Fong S, Malik PRV, Edginton AN. Quantifying breast milk intake by term and preterm infants for input into paediatric physiologically based pharmacokinetic models. Matern Child Nutr. 2020;16(2):e12938–e.
Maharaj AR, Barrett JS, Edginton AN. A workflow example of PBPK modeling to support pediatric research and development: case study with lorazepam. AAPS J. 2013;15(2):455–64.
Yuen WC, Peck AW. Lamotrigine pharmacokinetics: oral and IV infusion in man. Br J Clin Pharmacol. 1988;26:242P.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–57.
Berg M, Welty TE, Gidal BE, Diaz FJ, Krebill R, Szaflarski JP, et al. Bioequivalence Between Generic and Branded Lamotrigine in People With Epilepsy: The EQUIGEN Randomized Clinical Trial. JAMA Neurol. 2017;74(8):919–26.
Ebert U, Thong NQ, Oertel R, Kirch W. Effects of rifampicin and cimetidine on pharmacokinetics and pharmacodynamics of lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2000;56(4):299–304.
Gidal BE, Sheth R, Parnell J, Maloney K, Sale M. Evaluation of VPA dose and concentration effects on lamotrigine pharmacokinetics: implications for conversion to lamotrigine monotherapy. Epilepsy Res. 2003;57(2-3):85–93.
Birnbaum AK, Kriel RL, Burkhardt RT, Remmel RP. Rectal absorption of lamotrigine compressed tablets. Epilepsia. 2000;41(7):850–3.
Birnbaum AK, Kriel RL, Im Y, Remmel RP. Relative bioavailability of lamotrigine chewable dispersible tablets administered rectally. Pharmacotherapy. 2001;21(2):158–62.
Burger DM, Huisman A, Van Ewijk N, Neisingh H, Van Uden P, Rongen GA, et al. The effect of atazanavir and atazanavir/ritonavir on UDP-glucuronosyltransferase using lamotrigine as a phenotypic probe. Clin Pharmacol Ther. 2008;84(6):698–703.
Fillastre JP, Taburet AM, Fialaire A, Etienne I, Bidault R, Singlas E. Pharmacokinetics of lamotrigine in patients with renal impairment: influence of haemodialysis. Drugs Exp Clin Res. 1993;19(1):25–32.
Marcellin P, de Bony F, Garret C, Altman C, Boige V, Castelnau C, et al. Influence of cirrhosis on lamotrigine pharmacokinetics. Br J Clin Pharmacol. 2001;51(5):410–4.
Srichaiya A, Longchoopol C, Oo-Puthinan S, Sayasathid J, Sripalakit P, Viyoch J. Bioequivalence of generic lamotrigine 100-mg tablets in healthy Thai male volunteers: a randomized, single-dose, two-period, two-sequence crossover study. Clin Ther. 2008;30(10):1844–51.
van Luin M, Colbers A, Verwey-van Wissen CP, van Ewijk-Beneken-Kolmer EW, van der Kolk M, Hoitsma A, et al. The effect of raltegravir on the glucuronidation of lamotrigine. J Clin Pharmacol. 2009;49(10):1220–7.
Hermann R, Knebel NG, Niebch G, Richards L, Borlak J, Locher M. Pharmacokinetic interaction between retigabine and lamotrigine in healthy subjects. Eur J Clin Pharmacol. 2003;58(12):795–802.
Incecayir T, Agabeyoglu I, Gucuyener K. Comparison of plasma and saliva concentrations of lamotrigine in healthy volunteers. Arzneimittel-Forschung. 2007;57(8):517–21.
Wootton R, Soul-Lawton J, Rolan PE, Sheung CT, Cooper JD, Posner J. Comparison of the pharmacokinetics of lamotrigine in patients with chronic renal failure and healthy volunteers. Br J Clin Pharmacol. 1997;43(1):23–7.
Depot M, Powell JR, Messenheimer JA Jr, Cloutier G, Dalton MJ. Kinetic effects of multiple oral doses of acetaminophen on a single oral dose of lamotrigine. Clin Pharmacol Ther. 1990;48(4):346–55.
Edginton AN, Schmitt W, Voith B, Willmann S. A mechanistic approach for the scaling of clearance in children. Clin Pharmacokinet. 2006;45(7):683–704.
Edginton AN, Schmitt W, Willmann S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet. 2006;45(10):1013–34.
Willmann S, Höhn K, Edginton A, Sevestre M, Solodenko J, Weiss W, et al. Development of a physiology-based whole-body population model for assessing the influence of individual variability on the pharmacokinetics of drugs. J Pharmacokinet Pharmacodyn. 2007;34(3):401–31.
Badée J, Qiu N, Collier AC, Takahashi RH, Forrest WF, Parrott N, et al. Characterization of the ontogeny of hepatic UDP-glucuronosyltransferase enzymes based on glucuronidation activity measured in human liver microsomes. J Clin Pharmacol. 2019;59(Suppl 1):S42–s55.
Miyagi SJ, Collier AC. Pediatric development of glucuronidation: the ontogeny of hepatic UGT1A4. Drug Metab Dispos. 2007;35(9):1587–92.
U.S. Food & Drug Administration. LAMICITAL (Lamotrigine) Label. 2015.
Nordmo E, Aronsen L, Wasland K, Småbrekke L, Vorren S. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43(11):1893–7.
Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000;41(6):709–13.
Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. 2002. Report No.: 0146-6453 (Print) 0146-6453 Contract No.: 3-4.
Jann MW, Hon YY, Shamsi SA, Zheng J, Awad EA, Spratlin V. Lack of pharmacokinetic interaction between lamotrigine and olanzapine in healthy volunteers. Pharmacotherapy. 2006;26(5):627–33.
Gastrup S, Stage TB, Fruekilde PB, Damkier P. Paracetamol decreases steady-state exposure to lamotrigine by induction of glucuronidation in healthy subjects. Br J Clin Pharmacol. 2016;81(4):735–41.
Theis JG, Sidhu J, Palmer J, Job S, Bullman J, Ascher J. Lack of pharmacokinetic interaction between oxcarbazepine and lamotrigine. Neuropsychopharmacology. 2005;30(12):2269–74.
Vauzelle-Kervroëdan F, Rey E, Cieuta C, Pariente-Khayat A, Pons G. D′Athis P, et al. Influence of concurrent antiepileptic medication on the pharmacokinetics of lamotrigine as add-on therapy in epileptic children. Br J Clin Pharmacol. 1996;41(4):325–30.
Chen C, Casale EJ, Duncan B, Culverhouse EH, Gilman J. Pharmacokinetics of lamotrigine in children in the absence of other antiepileptic drugs. Pharmacotherapy. 1999;19(4):437–41.
Fotopoulou C, Kretz R, Bauer S, Schefold JC, Schmitz B, Dudenhausen JW, et al. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85(1):60–4.
Newport DJ, Pennell PB, Calamaras MR, Ritchie JC, Newman M, Knight B, et al. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122(1):e223–31.
Tomson T, Ohman I, Vitols S. Lamotrigine in pregnancy and lactation: a case report. Epilepsia. 1997;38(9):1039–41.
Bedussi F, Relli V, Faraoni L, Eleftheriou G, Giampreti A, Gallo M, et al. Normocytic Normochromic anaemia and asymptomatic neutropenia in a 40-Day-old infant breastfed by an epileptic mother treated with lamotrigine: Infant’s adverse drug reaction. J Paediatr Child Health. 2018;54(1):104–5.
Liporace J, Kao A, D'Abreu A. Concerns regarding lamotrigine and breast-feeding. Epilepsy Behav. 2004;5(1):102–5.
Argikar UA, Remmel RP. Variation in glucuronidation of lamotrigine in human liver microsomes. Xenobiotica. 2009;39(5):355–63.
Ladumor MK, Thakur A, Sharma S, Rachapally A, Mishra S, Bobe P, et al. A repository of protein abundance data of drug metabolizing enzymes and transporters for applications in physiologically based pharmacokinetic (PBPK) modelling and simulation. Sci Rep. 2019;9(1):9709.
Fríguls B, Joya X, García-Algar O, Pallás CR, Vall O, Pichini S. A comprehensive review of assay methods to determine drugs in breast milk and the safety of breastfeeding when taking drugs. Anal Bioanal Chem. 2010;397(3):1157–79.
Anderson PO, Manoguerra AS, Valdés V. A Review of Adverse Reactions in Infants From Medications in Breastmilk. Clin Pediatr. 2016;55(3):236–44.
Morin C, Chevalier I. Severe hypernatremic dehydration and lower limb gangrene in an infant exposed to lamotrigine, aripiprazole, and sertraline in breast milk. Breastfeed Med. 2017;12(6):377–80.
Acknowledgements
• Canadian Institutes of Health Research (CIHR); Project Grant; Award Number: PJT-159782
• Canadian Institutes of Health Research (CIHR); Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Award (CGS-D), a Canada Graduate Scholarship to Honour Nelson Mandela; Award Number: DF2-171445
Author information
Authors and Affiliations
Contributions
I, Andrea Edginton, am contributing this section to highlight the work of the authors in this research that directly relates to the health of women and children. This important work has been championed by women as well as men. Authorship of this manuscript highlights our combined efforts to bring clarity to breastfeeding mothers and clinicians during an exceptionally vulnerable time in women’s lives. Our male co-author Shinya Ito, has spent a career focused on discovery and education in pediatric clinical pharmacology including lactation and infant exposure to medications and we most certainly consider him a champion of the cause. Julie Autmizguine, a newer independent investigator, is bringing equity to children through her research that will improve health outcomes through investigation of appropriate medication use in this population. My role in this work, and throughout my career, is in integrating the vast body of knowledge discovered by others to answer relevant clinical questions through the use of modeling and simulation. My role is also to inspire and train the next generation of knowledge translators and the first author, Cindy Yeung, is one such trainee. She brings an expertise in systematic review and clinical care guideline development from her MSc degree and we are privileged to have her extend her skill set to PBPK modeling and risk assessment. While my and Cindy’s expertise differs from that of our clinical collaborators, the strength of our Canadian team is indeed in our divergent expertise. Our varied skills, motivations, backgrounds, and beliefs allow for a problem assessment that is far richer than any one of us alone could achieve.
Corresponding author
Additional information
Guest Editors: Diane Burgess, Marilyn Morris and Meena Subramanyam
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
"The original online version of this article was revised to update Table I, and to delete a paragraph from the “Results” section." plus the same explanatory text of the problem as in the erratum/correction article.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yeung, C.H.T., Ito, S., Autmizguine, J. et al. Incorporating Breastfeeding-Related Variability with Physiologically Based Pharmacokinetic Modeling to Predict Infant Exposure to Maternal Medication Through Breast Milk: a Workflow Applied to Lamotrigine. AAPS J 23, 70 (2021). https://doi.org/10.1208/s12248-021-00599-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12248-021-00599-5