Abstract
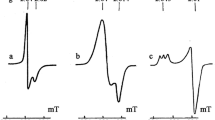
ESR spectroscopy coupled to the spin trapping technique was used to evaluate the generation of radical species arising from the ferrous ion induced decomposition of tert-butyl hydroperoxide (’BuOOH) in methylene chloride. We report here that N-tert-butyl-α-phenylinitrone (PBN) can trap peroxyl radicals generated in the ferrous ion induced breakdown of high concentration of ’BuOOH (IM) at room temperature, the radical adduct being stable under the light. The peroxyl radical formation was demonstrated by direct ESR measurements at 77K. In contrast, alkoxyl and methyl radicals were trapped only in the presence of low hydroperoxide concentration (ImM). In order to measure the hyperfine splitting constants (hfsc) of the PBN-methyl adduct spectra were obtained in the presence of diphenylamine (DPA) or 2,6-di-tert-butyl-4-methylphenol (BHT), which quenched the alkoxyl radical. For this latter radical, the hfsc were calculated by computer simulation. A mechanism for a direct interaction between DPA and the alkoxyl radical is presented. DPA quenched the peroxyl radical in the reaction of high hydroperoxide concentrations, with the concomitant generation of a DPA nitrogen-based radical.
Similar content being viewed by others
References
B. Kalyanaraman, C. Mottley, and R.P. Mason, J. Biol. Chem. 258, 3855 (1983).
W. Chamulitrat, N. Takahashi, and R.P. Mason, J. Biol. Chem. 264, 7889 (1989).
M.V. Merrit and R.A. Johnson, J. Am. Chem. Soc., 99, 3713 (1977).
E. Niki, S. Yokoi, J. Tsuchiya, and Y. Kamiya, J. Am. Chem. Soc. 105, 1498 (1983).
N. Ohto, E. Niki, and Y. Kamiya, J. Chem. Soc. Perkin Trans. II 2, 1770 (1977).
A.N. Osipov, V.M. Savov, A.V. Yachyaev, V.E. Zubarev, O.A. Azizova, V.E. Kagan, and Y.A. Vladimirov, Biofizika 29, 533 (1984).
J.A. Howard and J.C. Tait, Can. J. Chem. 56, 176 (1978).
M.J. Davies and T.F. Slater, Biochem. J. 245, 167 (1987).
W.T. Dixon and R.O.C. Norman, J. Chem. Soc. 3119 (1963).
M.F.R. Mulcahy, J.R. Stevens, and J.C. Ward, Aust. J. Chem. 18, 1177 (1965).
J.A. Weil and J.K. Anderson, J. Chem. Soc. 5567 (1965).
K.M. Schaich and D.C. Borg. In: M.G. Simic and M. Karel (Eds.), Autoxidation in Food and Biological Systems, Plenum Press, New York, 1980, p. 45.
E. Finkelsten, G.M. Rosen, E.J. Rauckman, and J. Paxton, Mol. Pharmacol. 16, 676 (1979).
W.A. Pryor and C.K. Govindan. J. Org. Chem. 46, 4679 (1981).
W.A. Pryor, C.K. Govindan, and D.F. Church, J. Am. Chem. Soc., 104, 7563 (1982).
W. Bors, C. Michel, and M. Saran, Bull. Europ. Physiopath. Resp. (suppl.), 17, 13 (1981)
J.R. Thomas and C.A. Tolman, J. Am. Chem. Soc. 84, 2930 (1962).
J.R. Thomas, J. Am. Chem. Soc. 82, 5955 (1960).
E.G. Janzen and I.G. Lopp, J. Phys. Chem. 76, 2056 (1972).
R. Hoskins, J. Chem. Phys., 25, 788 (1956).
A.N. Kuznetsov and V.A. Radtsig. Chem. Phys. Lett., 17, 377 (1972).
M.C.R. Symons, E. Albano, T.F. Slater, and A. Tomasi, J. Chem. Soc. Faraday Trans. I 78, 2205 (1982).
M.J. Davies and T.F. Slater, Biochem. J. 240, 789 (1986).
W. Bors, D. Tait, C. Michel, M. Saran, and M. Erben-Russ, Isr. J. Chem. 24, 17 (1984).
E.G. Janzen, D.E. Nutter, E.-R. Davis, B.J. Blackburn, J.L. Poyer, and P.B. McCay, Can. J. Chem. 56, 2237 (1978).
M.J. Davies and T.F. Slater, Chem.-Biol. Interact. 58, 137 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Iannone, A., Tomasi, A. & Canfield, L.M. Generation of N-tert-butyl-α-phenylnitrone radical adducts in iron breakdown of tert-butyl-hydroperoxide. Res. Chem. Intermed. 22, 469–479 (1996). https://doi.org/10.1163/156856796X00674
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856796X00674