Abstract
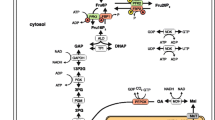
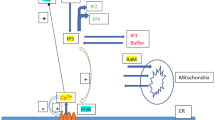
The liver’s primary function is to integrate various signals to retain stable blood glucose levels. Glycogenolysis, gluconeogenesis, and other metabolic processes are regulated by circulating hormones via calcium-dependent signaling. In this paper, the influence of calcium concentration on glycogen phosphorylase in hepatocytes is investigated utilizing a numerical approach. The system comprising two nonlinear reaction-diffusion equations for calcium and \(IP_{3}\), respectively, has been coupled to propose a mathematical model. The temporal equation of the fraction of active glycogen phosphorylase (\(\phi\)) is also incorporated into the model. The finite volume and the Crank Nicolson methods are implemented along spatial and temporal dimensions, while the Gauss-Seidel method is employed to simplify the resulting nonlinear equations. The impact of calcium influx, EGTA buffer concentration, SERCA pump rate constant, and leak flux constant on the \(\phi\) has been studied. It has been observed that interdependent calcium and \(IP_{3}\) dynamics have a crucial role in controlling the blood glucose level. Any dysregulation of calcium and \(IP_{3}\) processes can lead to the dysregulation of glycogen phosphorylase. This may lead to hyperglycemia or hypoglycemia.
Graphical abstract








Similar content being viewed by others
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
P.V. Roder, B. Wu, Y. Liu, W. Han, Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 48(3), e219 (2016). https://doi.org/10.1038/emm.2016.6
Z. Fu, E.R. Gilbert, D. Liu, Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 9(1), 25–53 (2013)
E. Mann, M. Sunni, M.D. Bellin, Secretion of insulin in response to diet and hormones. Pancreapedia The Exocrine Pancreas Knowledge Base (2020). https://doi.org/10.3998/panc.2020.16
F.L. Bygrave, A. Benedetti, Calcium: its modulation in liver by cross-talk between the actions of glucagon and calcium-mobilizing agonists. Biochem. J. 296(1), 1–14 (1993). https://doi.org/10.1042/bj2960001
S.R. Nagarajan, M. Paul-Heng, J.R. Krycer, D.J. Fazakerley, A.F. Sharland, A.J. Hoy, Lipid and glucose metabolism in hepatocyte cell lines and primary mouse hepatocytes: a comprehensive resource for in vitro studies of hepatic metabolism. Am. J. Physiol-Endocrinol Metab. 316(4), E578–E589 (2019). https://doi.org/10.1152/ajpendo.00365.2018
M.J. Berridge, Elementary and global aspects of calcium signalling. J. Physiol. 499(2), 291–306 (1997). https://doi.org/10.1113/jphysiol.1997.sp021927
N.M. Woods, K.R. Cuthbertson, P.H. Cobbold, Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319(6054), 600–602 (1986). https://doi.org/10.1038/319600a0
M.H. Nathanson, A.D. Burgstahler, M.B. Fallon, Multistep mechanism of polarized \(Ca^{2+}\) wave patterns in hepatocytes. Am. J. Physiol-Gastrointest Liver Physiol. 267(3), G338–G349 (1994). https://doi.org/10.1152/ajpgi.1994.267.3.G338
D. Gall, E. Baus, G. Dupont, Activation of the liver glycogen phosphorylase by \(Ca^{2+}\) oscillations: a theoretical study. J. Theor. Biol. 207(4), 445–454 (2000). https://doi.org/10.1006/jtbi.2000.2139
K.X. Walsh, D.M. Millikin, K.K. Schlender, E.M. Reimann, Calcium-dependent phosphorylation of glycogen synthase by phosphorylase kinase. J. Biol. Chem. 254, 6611–6616 (1979)
A.P. Thomas, D.C. Renard, T.A. Rooney, Spatial and temporal organization of calcium signalling in hepatocytes. Cell Calcium 12(2–3), 111–126 (1991). https://doi.org/10.1016/0143-4160(91)90013-5
G. Dupont, S. Swillens, C. Clair, T. Tordjmann, L. Combettes, Hierarchical organization of calcium signals in hepatocytes: from experiments to models Reveiw. Biochim Biophys Acta (BBA)-Molecular Cell Res. 1498(2), 134–152 (2000). https://doi.org/10.1016/s0167-4889(00)00090-2
J. Sneyd, Calcium buffering and diffusion: on the resolution of an outstanding problem. Biophys. J. 67(1), 4 (1994). https://doi.org/10.1016/S0006-3495(94)80448-5
G.D. Smith, A. Sherman, L. Dai, R.M. Miura, Asymptotic analysis of buffered calcium diffusion near a point source. SIAM J. Appl. Math. 61(5), 1816–1838 (2001)
M. Falcke, Buffers and oscillations in intracellular \(Ca^{2+}\) dynamics. Biophys. J . 84(1), 28–41 (2003). https://doi.org/10.1016/S0006-3495(03)74830-9
J.F. Dufour, I.M. Arias, T.J. Turner, Inositol 1, 4, 5-trisphosphate and calcium regulate the calcium channel function of the hepatic inositol 1, 4, 5-trisphosphate receptor. J. Biol. Chem. 272(5), 2675–2681 (1997). https://doi.org/10.1074/jbc.272.5.2675
N.L. Allbritton, T. Meyer, L. Stryer, Range of messenger action of calcium ion and inositol 1, 4, 5-trisphosphate. Science 258(5089), 1812–1815 (1992). https://doi.org/10.1126/science.1465619
J. Wagner, C.P. Fall, F. Hong, C.E. Sims, N.L. Allbritton, R.A. Fontanilla, I.I. Moraru, L.M. Loew, R. Nuccitelli, A wave of \(IP_{3}\) production accompanies the fertilization \(Ca^{2+}\) wave in the egg of the frog, xenopus laevis: theoretical and experimental support. Cell Calcium 35(5), 433–447 (2004). https://doi.org/10.1016/j.ceca.2003.10.009
M.L. Cardenas, A. Goldbeter, The glucose induced switch between glycogen phosphorylase and glycogen synthase in the liver: outlines of theoretical approach. J. Theor. Biol. 182, 421–426 (1996). https://doi.org/10.1006/jtbi.1996.0182
S. Tewari, K.R. Pardasani, Finite difference model to study the effects of Na+ influx on cytosolic \(Ca^{2+}\) diffusion. Int. J. Biol. Med. Sci. 1(4), 205–210 (2009)
S. Tewari, K.R. Pardasani, Finite element model to study two dimensional unsteady state cytosolic calcium diffusion in presence of excess buffers. IAENG Int. J. Appl. Math. 40(3), 108–112 (2010)
A. Jha, N. Adlakha, Two-dimensional finite element model to study unsteady state \(Ca^{2+}\) diffusion in neuron involving ER, leak and SERCA. Int. J. Biomath. 8(01), 1550002 (2015). https://doi.org/10.1142/S1793524515500023
B.K. Jha, H. Joshi, Chaos of calcium diffusion in Parkinson s infectious disease model and treatment mechanism via Hilfer fractional derivative. Math. Model. Numer. Simul. with Appl. 1, 84–94 (2021). https://doi.org/10.53391/mmnsa.2021.01.008
M. Kotwani, N. Adlakha, M. Mehta, Finite element model to study the effect of buffers, source amplitude and source geometry on spatiotemporal calcium distribution in fibroblast cell. J. Med. Imag. Health Inf. 4(6), 840–847 (2014). https://doi.org/10.1166/jmihi.2014.1328
N. Manhas, K.R. Pardasani, Mathematical model to study \(IP_{3}\) dynamics dependent calcium oscillations in pancreatic acinar cells. J. Med. Imag. Health Inf. 4(6), 874–880 (2014). https://doi.org/10.1166/jmihi.2014.1333
N. Manhas, K.R. Pardasani, Modelling mechanism of calcium oscillations in pancreatic acinar cells. J. Bioenerg. Biomembr. 46(5), 403–420 (2014). https://doi.org/10.1007/s10863-014-9561-0
N. Manhas, J. Sneyd, K.R. Pardasani, Modelling the transition from simple to complex \(Ca^{2+}\) oscillations in pancreatic acinar cells. J. Biosci. 39(3), 463–484 (2014). https://doi.org/10.1007/s12038-014-9430-3
P.A. Naik, K.R. Pardasani, One dimensional finite element model to study calcium distribution in oocytes in presence of VGCC, RyR and buffers. J. Med. Imag. Health Inf. 5(3), 471–476 (2015). https://doi.org/10.1166/jmihi.2015.1431
P.A. Naik, K.R. Pardasani, One dimensional finite element method approach to study effect of ryanodine receptor and SERCA pump on calcium distribution in oocytes. J. Multiscale Modell. 5(02), 135000715 (2013). https://doi.org/10.1142/S1756973713500078
P.A. Naik, K.R. Pardasani, Finite element model to study calcium distribution in oocytes involving voltage gated \(Ca^{2+}\) channel, ryanodine receptor and buffers. Alex. J. Med. 52(1), 43–49 (2016). https://doi.org/10.1016/j.ajme.2015.02.002
P.A. Naik, K.R. Pardasani, Three-dimensional finite element model to study effect of RyR calcium channel, ER leak and SERCA pump on calcium distribution in oocyte cell. Int. J. Comput. Methods. 16(01), 1850091 (2019). https://doi.org/10.1142/S0219876218500913
S. Panday, K.R. Pardasani, Finite element model to study effect of advection diffusion and \(Na^{+}/Ca^{2+}\) exchanger on \(Ca^{2+}\) distribution in Oocytes. J. Med. Imag. Health Inf. 3(3), 374–79 (2013). https://doi.org/10.1166/jmihi.2013.1184
S. Panday, K.R. Pardasani, Finite element model to study the mechanics of calcium regulation in oocyte. J. Mech. Med. Biol. 14(02), 1450022 (2014). https://doi.org/10.1142/S0219519414500225
P.A. Naik, J. Zu, Modeling and simulation of spatial-temporal calcium distribution in T lymphocyte cell by using a reaction-diffusion equation. J. Bioinform. Comput. Biol. 18(02), 2050013 (2020). https://doi.org/10.1142/S0219720020500134
P.A. Naik, Modeling the mechanics of calcium regulation in T lymphocyte: a finite element method approach. Int. J. Biomath. 13(05), 2050038 (2020). https://doi.org/10.1142/S1793524520500382
K.B. Pathak, N. Adlakha, Finite element model to study calcium signalling in cardiac myocytes involving pump, leak and excess buffer. J. Med. Imag. Health Inf. 5(4), 683–688 (2015). https://doi.org/10.1166/jmihi.2015.1443
Y.D. Jagtap, N. Adlakha, Finite volume simulation of two dimensional calcium dynamics in a hepatocyte cell involving buffers and fluxes. Commun. Math. Biol. Neurosci 2018, 1–16 (2018)
Y.D. Jagtap, N. Adlakha, Simulation of buffered advection diffusion of calcium in a hepatocyte cell. Math. Biol. Bioinf. 13, 609–619 (2018). https://doi.org/10.17537/2018.13.609
N. Singh, N. Adlakha, A mathematical model for interdependent calcium and inositol 1, 4, 5-trisphosphate in cardiac myocyte. Netw. Model Anal Health Inf. Bioinf. 8(1), 18 (2019). https://doi.org/10.1007/s13721-019-0198-0
Y.D. Jagtap, N. Adlakha, Numerical study of one-dimensional buffered advection-diffusion of calcium and \(IP_{3}\) in a hepatocyte cell. Netw. Model Anal Health Inf. Bioinf. 8, 25 (2019). https://doi.org/10.1007/s13721-019-0205-5
D.D. Dave, B.K. Jha, Analytically depicting the calcium diffusion for Alzheimer’s affected cell. Int. J. Biomath. 11(07), 1850088 (2018). https://doi.org/10.1142/S1793524518500882
B.K. Jha, N. Adlakha, M. Mehta, Two-dimensional finite element model to study calcium distribution in astrocytes in presence of excess buffer. Int. J. Biomath. 7(03), 1450031 (2014). https://doi.org/10.1142/S1793524514500314
H. Joshi, B.K. Jha, Chaos of calcium diffusion in Parkinson’s infectious disease model and treatment mechanism via Hilfer fractional derivative. Math. Modell. Numer. Simul. Appl. 1(2), 84–94 (2021). https://doi.org/10.53391/mmnsa.2021.01.008
H. Joshi, B.K. Jha, On a reaction-diffusion model for calcium dynamics in neurons with Mittag-Leffler memory. Eur. Phys. J. Plus 136(6), 1–15 (2021). https://doi.org/10.1140/epjp/s13360-021-01610-w
A. Pawar, K.R. Pardasani, Effects of disorders in interdependent calcium and \(IP_{3}\) dynamics on nitric oxide production in a neuron cell. Eur. Phys. J. Plus 137, 543 (2022). https://doi.org/10.1140/epjp/s13360-022-02743-2
A. Pawar, K.R. Pardasani, Simulation of disturbances in interdependent calcium and \(\beta\)-amyloid dynamics in the nerve cell. Eur. Phys. J. Plus. 137, 960 (2022). https://doi.org/10.1140/epjp/s13360-022-03164-x
P.J. Bartlett, L.D. Gaspers, N. Pierobon, A.P. Thomas, Calcium-dependent regulation of glucose homeostasis in the liver. Cell Calcium 55(6), 306–16 (2014). https://doi.org/10.1016/j.ceca.2014.02.007
I.V. Dokukina, M.V. Yamashev, E.A. Samarina, O.M. Tilinova, E.A. Grachev, Calcium-dependent insulin resistance in hepatocytes: mathematical model. J. Theor. Biol. 522, 110684 (2021). https://doi.org/10.1016/j.jtbi.2021.110684
J. Keener, J. Sneyd, Mathematical Physiology: I: Cellular Physiology (Springer Science & Business Media, UK, 2010)
C.P. Fall, Computational Cell Biology: Interdisciplinary Applied Mathematics (Springer-Verlag, New York Incorporated, 2002)
T.A. Rooney, E.J. Sass, A.P. Thomas, Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J. Biol. Chem. 265(18), 10792–10796 (1990)
S.A. Brown, F. Morgan, J. Watras, L.M. Loew, Analysis of phosphatidylinositol-4, 5-bisphosphate signaling in cerebellar purkinje spines. Biophys. J. 95(4), 1795–1812 (2008). https://doi.org/10.1529/biophysj.108.130195
H.K. Versteeg (Henk Kaarle), An introduction to computational fluid dynamics : the finite volume method. (Harlow, Essex, England ; Longman Scientific & Technical :New York : Wiley, (1995)) pp. 115-132
A. Politi, L.D. Gaspers, A.P. Thomas, T. Hofer, Models of \(IP_{3}\) and \(Ca^{2+}\) oscillations: frequency encoding and identification of underlying feedbacks. Biophys. J . 90(9), 3120–3133 (2006). https://doi.org/10.1529/biophysj.105.072249
Author information
Authors and Affiliations
Corresponding author
Appendix: model equations summary
Appendix: model equations summary
Solving space integration of Eq. (23) we get,
The time integral is simplified by using weighted parameter \(\Psi\), where \(\Psi \in \left[ 0, 1 \right]\).
where the 0 super scripted values of C represents initial concentrations. The Crank Nicolson method is employed by substituting \(\Psi = 1/2\). The hepatocyte cell is assumed to be discretized by 24 nodes. Equation (29) is arranged at ‘\(j^{th}\)’ iteration in the following form,
where,
and nonlinear term of \(Ca^{2+}\) is given by,
First boundary condition can be incorporated by setting, \(C_{W}=\sigma _{Ca}\) and \(a_{W}=0\),
where
Second boundary condition can be incorporated by setting \(C_{\infty }=0.1 \,\mu M\) and \(a_{E}=0\),
where,
Integrating Eq. (24) along spatial dimension we get,
The time integral is simplified using the weighted parameter \(\Psi\), where \(\Psi \in \left[ 0, 1 \right]\).
where the 0 super scripted values of P represents initial concentrations. The Crank Nicolson method is employed by substituting \(\Psi = 1/2\). Equation (34) is arranged in the following form for all internal nodes at ‘\(j^{th}\)’ iteration,
where,
and nonlinear term of \(IP_{3}\) is given by,
First boundary condition can be incorporated by setting, \((P_{c})_{E}=\sigma _{P}\) and \(b_{E}=0\),
where
Second boundary condition can be incorporated by setting \(P_{\infty }=10 \mu M\) and \(a_{W}=0\),
where
All discretized equations obtained from Eqs. (30)– (32) and Eqs. (35)– (37) are expressed in matrix form as follows;
where [L] is diagonal block matrix, \(\begin{bmatrix} C \\ P_{c}\end{bmatrix}\) is calcium and \(IP_{3}\) concentration vector, [M] is constant vector. At each time step Eq. (38) is solved by using Gauss-Seidel’s method to get desired solution vector \(\begin{bmatrix} C \\ P_{c} \end{bmatrix}_{24\times 1}\)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jagtap, Y., Adlakha, N. Numerical model of hepatic glycogen phosphorylase regulation by nonlinear interdependent dynamics of calcium and \(IP_{3}\). Eur. Phys. J. Plus 138, 399 (2023). https://doi.org/10.1140/epjp/s13360-023-03961-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-023-03961-y