Abstract
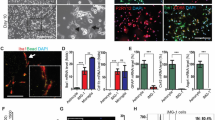
SIM-A9 is a line of spontaneously immortalized mouse microglial cells obtained from the brain of newborn C57BL/6 mice. The purpose of this work is to characterize the microglia of the SIM-A9 mouse line on the basis of the ratio of cells with the phenotype of resting and activated microglia in culture and to analyze the expression of markers of CD133 stem (progenitor) cells and nestin, which are receptors for growth factors CSF-1R and EGFR, as well as to analyze the karyotype of this line. Light microscopy and immunocytochemistry combined with flow cytometry and RT–PCR were used to analyze the morphology, phenotype, and expression level of pro-inflammatory cytokine genes, and the mFISH method was used to analyze the karyotype. It has been shown for the first time that SIM-A9 cells express a high level of TSPO protein and CD68, CD11b, and CD45high markers on the surface membrane of cells, which corresponds to the phenotype of activated microglia. Despite this, the cells of the line respond with additional activation in response to lipopolysaccharide stimulation, which leads to an increase in the expression of pro-inflammatory cytokine genes IL-1β, TNFα, and IL-6 and the formation of a high level of active oxygen and nitrogen metabolites. SIM-A9 cells were shown to express markers of stem and progenitor cells CD133+ and nestin, which allows us to consider them as early poorly differentiated progenitor cells, despite their phenotype corresponding to activated microglia. It was also found that SIM-A9 cells express receptors of the two growth factors CSF-1 and EGF, CSF-1R and EGFR, which indicates the possibility of stimulation of SIM-A9 cell proliferation by two alternative mechanisms under the action of corresponding factors. SIM-A9 cells have a hypotetraploid karyotype with a large number of structural and quantitative chromosome anomalies.








Similar content being viewed by others
REFERENCES
Moskaleva, E.Yu., Rodina, A.V., Semochkina, Ju.P., and Vysotskaya, O.V., Analysis of neurons damage and level of neuroinflammation late after γ-irradiation of mice head at different doses, Radiats. Biol. Radioekol., 2022, vol. 62, no. 2, p. 171. https://doi.org/10.31857/S0869803122020059
Novikov, V.E., Levchenkova, O.S., and Pozhilova, Y.V., Role of reactive oxygen species in cell physiology and pathology and their pharmacological regulation, Obzory Klin. Farmakol. Lekarstv. Ter., 2014, vol. 12, no. 4, p. 13. https://doi.org/10.17816/RCF12413-21
Askew, K., Li, K., Olmos-Alonso, A., Garcia-Moreno, F., Liang, Y., Richardson, P., Tipton, T., Chapman, M.A., Riecken, K., Beccari, S., Sierra, A., Molnár, Z., Cragg, M.S., Garaschuk, O., Perry, V.H., and Gomez-Nicola, D., Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain, Cell Rep., 2017, vol. 18, p. 391. https://doi.org/10.1016/j.celrep.2016.12.041
Bachiller, S., Jiménez-Ferrer, I., Paulus, A., Yang, Y., Swanberg, M., Deierborg, T., and Boza-Serrano, A., Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response, Front. Cell Neurosci., 2018, vol. 12, p. 488. https://doi.org/10.3389/fncel.2018.00488
Becher, B. and Antel, J.P., Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia, Glia, 1996, vol. 18, p. 1. https://doi.org/10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6
Bennett, M.L., Bennett, F.C., Liddelow, S.A., Ajami, B., Zamanian, J.L., Fernhoff, N.B., Mulinyawe, S.B., Bohlen, C.J., Adil, A., Tucker, A., Weissman, I.L., Chang, E.F., Li, G., Grant, G.A., Hayden Gephart, M.G., and Barres, B.A., New tools for studying microglia in the mouse and human CNS, Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, p. 1738. https://doi.org/10.1073/pnas.1525528113
Bernal, A. and Arranz, L., Nestin-expressing progenitor cells: function, identity and therapeutic implications, Cell. Mol. Life Sci., 2018, vol. 75, p. 2177. https://doi.org/10.1007/s00018-018-2794-z
Blasi, E., Barluzzi, R., Bocchini, V., Mazzolla, R., and Bistoni, F., Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus, J. Neuroimmunol., 1990, vol. 27, p. 229. https://doi.org/10.1016/0165-5728(90)90073-V
Bohnert, S., Seiffert, A., Trella, S., Bohnert, M., Distel, L., Ondruschka, B., and Monoranu, C.M., TMEM119 as a specific marker of microglia reaction in traumatic brain injury in postmortem examination, Int. J. Legal Med., 2020, vol. 134, p. 2167. https://doi.org/10.1007/s00414-020-02384-z
Bonsack, F. and Sukumari-Ramesh, S., TSPO: an evolutionarily conserved protein with elusive functions, Int. J. Mol. Sci., 2018, vol. 19, p. 1694. https://doi.org/10.3390/ijms19061694
Cornforth, M.N., Analyzing radiation-induced complex chromosome rearrangements by combinatorial painting, Radiat. Res., 2001, vol. 155, p. 643. https://doi.org/10.1007/978-1-4939-9432-8_15
Coniglio, S.J., Eugenin, E., Dobrenis, K., Stanley, E.R., West, B.L., Symons, M.H., and Segall, J.E., Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling, Mol. Med., 2012, vol. 18, p. 519. https://doi.org/10.2119/molmed.2011.00217
Coskun, V., Wu, H., Blanchi, B., Tsao, S., Kim, K., Zhao, J., Biancotti, J.C., Hutnick, L., Krueger, R.C., Jr., Fan, G., de Vellis, J., and Sun, Y.E., CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain, Proc. Natl. Acad. Sci. U. S. A., 2008, vol. 105, p. 1026. https://doi.org/10.1073/pnas.0710000105
Douglas, M.R., Morrison, K.C., Jacques, S.J., Leadbeater, W.E., Gonzalez, A.M., Berry, M., Logan, A., and Ahmed, Z., Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth, Brain, 2009, vol. 132, p. 3102. https://doi.org/10.1093/brain/awp240
Elmore, M.R., Najafi, A.R., Koike, M.A., Dagher, N.N., Spangenberg, E.E., Rice, R.A., Kitazawa, M., Matusow, B., Nguyen, H., West, B.L., and Green, K.N., Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain, Neuron, 2014, vol. 82, p. 380. https://doi.org/10.1016/j.neuron.2014.02.040
Eyo, U.B. and Dailey, M.E., Microglia: key elements in neural development, plasticity, and pathology, J. Neuroimmune Pharmacol., 2013, vol. 8, p. 494. https://doi.org/10.1007/s11481-013-9434-z
Fischer, O.M., Hart, S., and Ullrich, A., Dissecting the epidermal growth factor receptor signal transactivation pathway, Methods Mol. Biol., 2006, vol. 327, p. 85. https://doi.org/10.1385/1-59745-012-X:85
Green, K.N., Crapser, J.D., and Hohsfield, L.A., To kill a microglia: a case for CSF1R inhibitors, Trends Immunol., 2020, vol. 41, p. 771. https://doi.org/10.1016/j.it.2020.07.001
Hagan, N., Kane, J.L., Grover, D., Woodworth, L., Madore, C., Saleh, J., Sancho, J., Liu, J., Li, Y., Proto, J., Zelic, M., Mahan, A., Kothe, M., Scholte, A.A., and Fitzgerald, M., CSF1R signaling is a regulator of pathogenesis in progressive MS, Cell Death Dis., 2020, vol. 11, p. 904. https://doi.org/10.1038/s41419-020-03084-7
Han, J., Chitu, V., Stanley, E.R., Wszolek, Z.K., Karrenbauer, V.D., and Harris, R.A., Inhibition of colony stimulating factor-1 receptor (CSF-1R) as a potential therapeutic strategy for neurodegenerative diseases: opportunities and challenges, Cell. Mol. Life Sci., 2022, vol. 79, p. 219. https://doi.org/10.1007/s00018-022-04225-1
Huang, Y., Xu, Z., and Xiong, S., Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion, Nat. Neurosci., 2018, vol. 21, p. 530. https://doi.org/10.1038/s41593-018-0090-8
Jenkins, S.J., Ruckerl, D., Thomas, G.D., Hewitson, J.P., Duncan, S., Brombacher, F., Maizels, R.M., Hume, D.A., and Allen, J.E., IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1, J. Exp. Med., 2013, vol. 210, p. 2477. https://doi.org/10.1084/jem.20121999
Jones, S. and Rappoport, J.Z., Interdependent epidermal growth factor receptor signalling and trafficking, Int. J. Biochem. Cell. Biol., 2014, vol. 51, p. 23. https://doi.org/10.1016/j.biocel.2014.03.014
Jurga, A.M., Paleczna, M., and Kuter, K.Z., Overview of general and discriminating markers of differential microglia phenotypes, Front. Cell Neurosci., 2020, vol. 14, p. 198. https://doi.org/10.3389/fncel.2020.00198
Kalm, M., Andreasson, U., Björk-Eriksson, T., Zetterberg, H., Pekny, M., Blennow, K., Pekna, M., and Blomgren, K., C3 deficiency ameliorates the negative effects of irradiation of the young brain on hippocampal development and learning, Oncotarget, 2016, vol. 7, p. 19382. https://doi.org/10.18632/oncotarget.8400
Lei, F., Cui, N., Zhou, C., Chodosh, J., Vavvas, D.G., and Paschalis, E.I., CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages, Proc. Natl. Acad. Sci. U. S. A., 2020, vol. 22, p. 23336. https://doi.org/10.1073/pnas.1922788117
Lively, S. and Schlichter, L.C., Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+TNFα) and reprogramming by resolving cytokines (IL-4, IL-10), Front. Cell Neurosci., 2018, vol. 12, p. 215. https://doi.org/10.3389/fncel.2018.00215
Liu, G.J., Middleton, R.J., Hatty, C.R., Kam, W.W., Chan, R., Pham, T., Harrison-Brown, M., Dodson, E., Veale, K., and Banati, R.B., The 18 kDa translocator protein, microglia and neuroinflammation, Brain Pathol., 2014, vol. 24, p. 631. https://doi.org/10.1111/bpa.12196
Mansour, H.M., Fawzy, H.M., El-Khatib, A.S., and Khattab, M.M., Repurposed anti-cancer epidermal growth factor receptor inhibitors: mechanisms of neuroprotective effects in Alzheimer’s disease, Neural Regen. Res., 2022, vol. 17, p. 1913. https://doi.org/10.4103/1673-5374.332132
Michalczyk, K. and Ziman, M., Nestin structure and predicted function in cellular cytoskeletal organization, Histol. Histopathol., 2005, vol. 20, p. 665. https://doi.org/10.14670/HH-20.665
Muñoz-Garcia, J., Cochonneau, D., Télétchéa, S., Moranton, E., Lanoe, D., Brion, R., Lézot, F., Heymann, M.F., and Heymann, D., The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis, Theranostics, 2021, vol. 11, p. 1568. https://doi.org/10.7150/thno.50683
Nagamoto-Combs, K., Kulas, J., and Combs, C.K., A novel cell line from spontaneously immortalized murine microglia, J. Neurosci. Methods, 2014, vol. 15, p. 187. https://doi.org/10.1016/j.jneumeth.2014.05.021
Onyango, I.G., Jauregui, G.V., Čarná, M., Bennett, J.P., Jr., and Stokin, G.B., Neuroinflammation in Alzheimer’s disease, Biomedicines, 2021, vol. 9, p. 524. https://doi.org/10.3390/biomedicines9050524
Pannell, M., Economopoulos, V., Wilson, T.C., Kersemans, V., Isenegger, P.G., Larkin, J.R., Smart, S., Gilchrist, S., Gouverneur, V., and Sibson, N.R., Imaging of translocator protein upregulation is selective for pro-inflammatory polarized astrocytes and microglia, Glia, 2020, vol. 68, p. 280. https://doi.org/10.1002/glia.23716
Prater, K.E., Aloi, M.S., Pathan, J.L., Winston, C.N., Chernoff, R.A., Davidson, S., Sadgrove, M., McDonough, A., Zierath, D., Su, W., Weinstein, J.R., and Garden, G.A., A subpopulation of microglia generated in the adult mouse brain originates from prominin-1-expressing progenitors, J. Neurosci., 2021, vol. 41, p. 7942. https://doi.org/10.1523/JNEUROSCI.1893-20.2021
Qu, W.S., Tian, D.S., Guo, Z.B., Fang, J., Zhang, Q., Yu, Z.Y., Xie, M.J., Zhang, H.Q., Lü, J.G., and Wang, W., Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury, J. Neuroinflammation, 2012, vol. 9, p. 178. https://doi.org/10.1186/1742-2094-9-178
Qu, W.S., Liu, J.L., Li, C.Y., Li, X., Xie, M.J., Wang, W., and Tian, D.S., Rapidly activated epidermal growth factor receptor mediates lipopolysaccharide-triggered migration of microglia, Neurochem. Int., 2015, vol. 90, p. 85. https://doi.org/10.1016/j.neuint.2015.07.007
Ramprasad, M.P., Terpstra, V., Kondratenko, N., Quehenberger, O., and Steinberg, D., Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein, Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, p. 14833. https://doi.org/10.1073/pnas.93.25.14833
Sasaki, Y., Ohsawa, K., Kanazawa, H., Kohsaka, S., and Imai, Y., Iba1 is an actin-cross-linking protein in macrophages/microglia, Biochem. Biophys. Res. Commun., 2001, vol. 286, p. 292. https://doi.org/10.1006/bbrc.2001.5388
Stanley, E.R. and Chitu, V., CSF-1 receptor signaling in myeloid cells, Cold Spring Harb. Perspect. Biol., 2014, vol. 6, p. a021857. https://doi.org/10.1101/cshperspect.a021857
Stansley, B., Post, J., and Hensley, K., A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease, J. Neuroinflammation, 2012, vol. 9, p. 115. https://doi.org/10.1186/1742-2094-9-115
Takamori, Y., Mori, T., Wakabayashi, T., Nagasaka, Y., Matsuzaki, T., and Yamada, H., Nestin-positive microglia in adult rat cerebral cortex, Brain Res., 2009, vol. 1270, p. 10. https://doi.org/10.1016/j.brainres.2009.03.014
Waller, R., Baxter, L., Fillingham, D.J., Coelho, S., Pozo, J.M., Mozumder, M., Frangi, A.F., Ince, P.G., Simpson, J.E., and Highley, J.R., Iba-1-/CD68+ microglia are a prominent feature of age-associated deep subcortical white matter lesions, PLoS One, 2019, vol. 25, p. e0210888. https://doi.org/10.1371/journal.pone.0210888
Wohl, S.G., Schmeer, C.W., Friese, T., Witte, O.W., and Isenmann, S., In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo, PLoS One, 2011, vol. 6, p. e22408. https://doi.org/10.1371/journal.pone.0022408
Wong, A.M., Patel, N.V., Patel, N.K., Wei, M., Morgan, T.E., de Beer, M.C., de Villiers, W.J., and Finch, C.E., Macrosialin increases during normal brain aging are attenuated by caloric restriction, Neurosci. Lett., 2005, vol. 390, p. 76. https://doi.org/10.1016/j.neulet.2005.07.058
Yan, P., Wu, X., Liu, X., Cai, Y., Shao, C., and Zhu, G., A causal relationship in spinal cord injury rat model between microglia activation and EGFR/MAPK detected by overexpression of microRNA-325-3p, J. Mol. Neurosci., 2019, vol. 68, p. 181. https://doi.org/10.1007/s12031-019-01297-w
Yang, Y., Sun, Y., Hu, R., Yan, J., Wang, Z., Li, W., and Jiang, H., Morphine promotes microglial activation by upregulating the EGFR/ERK signaling pathway, PLoS One, 2021, vol. 16 P. e0256870. https://doi.org/10.1371/journal.pone.0256870
Funding
This work was supported by National Research Center “Kurchatov Institute” and the Joint Institute for Nuclear Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. Animals or human beings were not used in the experiments.
Additional information
Abbreviations: AFN—active form of nitrogen; ROS—reactive oxygen species; AChr—chromosomal aberration; LPS—lipopolysaccharide; SC—stem cells, EDTA—ethylenediaminetetraacetic acid; EGTA—ethylene glycol-di-(2-aminoethyl)-tetraacetic acid; CNS—central nervous system; CSF-1—colony stimulating factor-1; CSF-1R—CSF-1 receptor; EGF—epidermal growth factor; EGFR—EGF receptor; mFISH—multicolor fluorescent hybridization in situ; PBS—phosphate-buffered saline solution.
Rights and permissions
About this article
Cite this article
Shaposhnikova, D.A., Moskaleva, E.Y., Syomochkina, Y.P. et al. Characteristics of SIM-A9 Microglia Cells: New Data. Cell Tiss. Biol. 17, 503–516 (2023). https://doi.org/10.1134/S1990519X23050127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X23050127