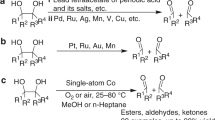
Abstract
The review deals with the development of a process for preparing cobalt catalysts for hydroformylation and carboalkoxylation. Common term oxo synthesis is used for these reactions. Theoretical principles of the classical synthesis of cobalt carbonyls are discussed, and the main directions of the modern development of oxo synthesis are considered. Much attention is paid to carboalkoxylation catalysts based on cobalt carbonyl complexes. The use of nitrogen bases as promoters of ethylene carboalkoxylation catalysts and the use of cobalt carbonyls on an inorganic support are analyzed. An approach to solving the problem of recycling oxo synthesis catalysts, alternative to heterogeneous catalysis, namely, thermoregulated phase-transfer cobalt catalysis, is described.











Similar content being viewed by others
REFERENCES
Patent DE 849548, Publ. 1938.
Wender, I., Sternberg, H.W., and Orchin, M., J. Am. Chem. Soc., 1953, vol. 75, no. 12, pp. 3041–3042. https://doi.org/10.1021/ja01108a528
Cornils, B., Herrmann, W.A., and Rasch, M., Angew. Chem. Int. Ed., 1994, vol. 33, no. 21, pp. 2144–2163. https://doi.org/10.1002/anie.199421441
Beller, M., Cornils, B., Frohning, C.D., and Kohlpaintner, C.W., J. Mol. Catal. A, 1995, vol. 104, no. 1, pp. 17–85. https://doi.org/10.1016/1381-1169(95)00130-1
Hood, D.M., Johnson, R.A., Carpenter, A.E., Younker, J.M., Vinyard, D.J., and Stanley, G.G., Science, 2020, vol. 367, no. 6477, pp. 542–548. https://doi.org/10.1126/science.aaw7742
Guo, J., Zhang, D., and Wang, X., ACS Catal., 2020, vol. 10, no. 22, pp. 13551–13559. https://doi.org/10.1021/acscatal.0c03161
Delolo, F.G., Yang, J., Neumann, H., dos Santos, E.N., Gusevskaya, E.V., and Beller, M., ACS Sustain. Chem. Eng., 2021, vol. 9, no. 14, pp. 5148–5154. https://doi.org/10.1021/acssuschemeng.1c00205
Takebayashi, S. and Fayzullin, R.R., Organometallics, 2021, vol. 40, no. 4, pp. 500–507. https://doi.org/10.1021/acs.organomet.0c00765
Hebrard, F. and Kalck, P., Chem. Rev., 2009, vol. 109, no. 9, pp. 4272–4282. https://doi.org/10.1021/cr8002533
de Vries, J.G., Hydroformylation of alkenes: Industrial applications, Science of Synthesis: C-1 Building Blocks in Organic Synthesis 1, van Leeuwen, P.W.N.M., Ed., 2014, pp. 193–227.
Stanley, G.G., Hydroformylation (OXO) catalysis, Kirk–Othmer Encyclopedia of Chemical Technology, Wiley, 2017, pp. 1–19. https://doi.org/10.1002/0471238961.1524150209121.a01.pub2
Hieber, W. and Scheclten, H., Z. Anorg. Allg. Chem., 1939, vol. 243, no. 2, pp. 145–163. https://doi.org/10.1002/zaac.19392430204
Wender, I., Greenfield, H., and Orchin, M., J. Am. Chem. Soc., 1951, vol. 73, no. 6, pp. 2656–2658. https://doi.org/10.1021/ja01150a069
Ercoli, R., Chini, P., and Massi-Mauri, M., Chim. Ind., 1959, vol. 41, pp. 132–135.
Chini, P., Chim. Ind., 1960, vol. 42, pp. 137–142.
Hieber, W. and Wiesboeck, R., Ber., 1958, vol. 91, no. 6, pp. 1146–1155. https://doi.org/10.1002/cber.19580910604
Patent US 2734922A, Publ. 1956.
Patent US 2757202A, Publ. 1956.
Patent GB 667093A, Publ. 1952.
Patent GB 679366A, Publ. 1952.
Patent GB 708441A, Publ. 1954.
Patent GB 740708A, Publ. 1955.
Patent FR 1076680A, Publ. 1954.
Patent US 2736750A, Publ. 1956.
Vigranenko, Yu.T. and Rybakov, V.A., Zh. Prikl. Khim., 1979, vol. 52, no. 10, pp. 2182–2185.
Vigranenko, Yu.T., Rybakov, V.A., Mukhenberg, K.M., Semenova, T.A., and Tarasov, B.P., Koord. Khim., 1989, vol. 15, no. 1, pp. 103–107.
Patent RU 2077948 C1, 1997.
Gankin, V.Yu. and Gurevich, G.S., Tekhnologiya oksosinteza (Oxo Synthesis Technology), Leningrad: Khimiya, 1981, pp. 112–120.
Rudkovskii, D.M., Trifel’, A.G., and Alekseeva, K.A., Khim. Prom–st., 1959, no. 8, pp. 652–658.
Wender, I., Levine, R., and Orchin, M., J. Am. Chem. Soc., 1950, vol. 72, no. 10, pp. 4375–4378. https://doi.org/10.1021/ja01166a012
Sternberg, H.W., Wender, J., Friedel, R.A., and Orchin, M., J. Am. Chem. Soc., 1953, vol. 75, no. 11, pp. 2717–2720. https://doi.org/10.1021/ja01107a050
Moore, E.J., Sullivan, J.M., and Norton, J.R., J. Am. Chem. Soc., 1986, vol. 108, no. 9, pp. 2257–2263. https://doi.org/10.1021/ja00269a022
Gorbunov, D.N., Nenasheva, M.V., and Kardashev, S.V., Russ. J. Appl. Chem., 2019, vol. 92, no. 8, pp. 1069−1076. https://doi.org/10.1134/S1070427219080032
Ziegler, T. and Versluis, L., Adv. Chem., 1992, vol. 230, pp. 75–93. https://doi.org/10.1021/ba-1992-0230.ch005
Sokolov, B.G., Katsnel’son, M.G., and Tarasov, B.P., Zh. Prikl. Khim., 1990, vol. 63, no. 9, pp. 2008–2013.
Henrici-Olive, G. and Olive, S., Coordination and Catalysis, Weinheim: Chemie, 1977.
Mirbach, М.F. and Mirbach, М.I., J. Mol. Catal., 1985, vol. 32, no. 1, pp. 59–75. https://doi.org/10.1016/0304-5102(85)85033-1
Tuba, R., Mika, L.T., Bodor, A., Pusztai, Z., Tóth, I., and Horváth, I.T., Organometallics, 2003, vol. 22, no. 8, pp. 1582–1584. https://doi.org/10.1021/om030058x
Milstein, D., Acc. Chem. Res., 1988, vol. 21, no. 11, pp. 428–434. https://doi.org/10.1021/ar00155a007
Patent RU 2727507 C1, 2020.
Patent RU 2756174 C1, 2021.
USSR Inventor’s Certificate no. 127250, 1958, Byull. Izobret., 1960, no. 7; Patent SU 127250, Publ. 1960.
Gankin, V.Yu., Gordina, N.Ya., Efimova, N.I., Krinkin, D.P., Rudkovskii, D.M., and Trifel’, A.G., Hydroformylation on fixed catalyst, Gidroformilirovanie (Hydroformylation), Imyanitov, N.S., Ed., Leningrad: Khimiya, 1972, pp. 90–93.
Gankin, V.Yu., Gordina, N.Ya., Krinkin, D.P., Rudkovskii, D.M., and Trifel’, A.G., Khim. Tekhnol. Topl. Masel, 1966, vol. 11, no. 4, pp. 8–10.
Bronshtein, Yu.E., Gankin, V.Yu., Krinkin, D.P., and Rudkovskii, D.M., Zh. Fiz. Khim., 1966, vol. 40, no. 7, pp. 1475–1482.
Kumar, R. and Chikkali, S.H., J. Organomet. Chem., 2022, vol. 960, ID 122231. https://doi.org/10.1016/j.jorganchem.2021.122231
Zhang, Y., Nagasaka, K., Qiu, X., and Tsubaki, N., Catal. Today, 2005, vol. 104, no. 1, pp. 48–54. https://doi.org/10.1016/j.cattod.2005.03.029
Hu, X., Shi, Y., Zhang, Y., Zhu, B., Zhang, S., and Huang, W., Catal. Commun., 2015, vol. 59, pp. 45–49. https://doi.org/10.1016/j.catcom.2014.09.043
Ahmed, M. and Sakthivel, A., J. Mol. Catal. A: Chemical, 2016, vol. 424, pp. 85–90. https://doi.org/10.1016/j.molcata.2016.08.016
Hertrich, M.F., Scharnagl, F.K., Pews-Davtyan, A., Kreyenschulte, C.R., Lund, H., Bartling, S., Jackstell, R., and Beller, M., Chem. Eur. J., 2019, vol. 25, no. 21, pp. 5534–5538. https://doi.org/10.1002/chem.201806282
Zhao, J., He, Y., Wang, F., Zheng, W., Huo, C., Liu, X., Jiao, H., Yang, Y., Li, Y., and Wen, X., ACS Catal., 2020, vol. 10, no. 2, pp. 914–920. https://doi.org/10.1021/acscatal.9b03228
Zhao, J., He, Y., Wang, F., Yang, Y., Zheng, W., Huo, C., Jiao, H., Yang, Y., Li, Y., and Wen, X., J. Catal., 2021, vol. 404, pp. 244–249. https://doi.org/10.1016/j.jcat.2021.09.031
Wei, B., Liu, X., Deng, Y., Hua, K., Chen, J., Wang, H., and Sun, Y., ACS Catal., 2021, vol. 11, no. 23, pp. 14319–14327. https://doi.org/10.1021/acscatal.1c04022
Wang, H., Yuan, H., Chen, X., Wang, X., Zhao, K., and Shi, F., J. Phys. Chem. C, 2022, vol. 126, no. 1, pp. 273−281. https://doi.org/10.1021/acs.jpcc.1c09309
Gong, H., Zhao, X., Qin, Y., Xu, W., Wei, X., Peng, Q., Ma, Y., Dai, S., An, P., and Hou, Z., J. Catal., 2022, vol. 408, pp. 245–260. https://doi.org/10.1016/j.jcat.2022.03.011
Patent US 3420898A, Publ. 1969.
Bungu, P.N. and Otto, S., Dalton Trans., 2007, no. 27, pp. 2876–2887. https://doi.org/10.1039/B702709E
Wiese, K.D. and Obst, D., Topics in Organometallic Chemistry, Beller, M., Ed., 2014, vol. 18, pp. 1–33.
Beller, M. and Krauter, J.G.E., J. Mol. Catal. A: Chemical, 1999, vol. 143, nos. 1–3, pp. 31–39. https://doi.org/10.1016/S1381-1169(98)00360-4
Haumann, M., Koch, H., and Schomacker, R., Catal. Today, 2003, vols. 79–80, pp. 43–49. https://doi.org/10.1016/S0920-5861(03)00041-5
Dabbawala, A., Parmar, D.U., Bajaj, H.C., and Jasra, R.V., J. Mol. Catal. A: Chemical, 2008, vol. 282, nos. 1–2, pp. 99–106. https://doi.org/10.1016/j.molcata.2007.11.026
Dabbawala, A.A., Parmar, J.N., Jasra, R.V., Bajaj, H.C., and Monflier, E., Catal. Commun., 2009, vol. 10, no. 14, pp. 1808–1812. https://doi.org/10.1016/j.catcom.2009.06.005
Dabbawala, A.A., Bajaj, H.C., Bricout, H., and Monflier, E., Appl. Catal. A: General, 2012, vols. 413–414, pp. 273–279. https://doi.org/10.1016/j.apcata.2011.11.021
Wu, D., Zhang, J., Wang, Y., Jiang, J., and Jin, Z., Appl. Organomet. Chem., 2012, vol. 26, no. 12, pp. 718–721. https://doi.org/10.1002/aoc.2916
Wu, D., Wang, Y., Li, G., Jiang, J., and Jin, Z., Catal. Commun., 2014, vol. 44, no. 10, pp. 54–56. https://doi.org/10.1016/j.catcom.2013.06.029
Roesle, P., Durr, C.J., Moller, Н.М., Cavallo, L., Caporaso, L., and Mecking, S., J. Am. Chem. Soc., 2012, vol. 134, no. 42, pp. 17696–17703. https://doi.org/10.1021/ja307411p
Zubiri, M.R.I., Clarke, M.L., Foster, D.F., Cole-Hamilton, D.J., Slawin, A.M.Z., and Woollins, J.D., J. Chem. Soc., Dalton Trans., 2001, no. 7, pp. 969–971. https://doi.org/10.1039/B101656N
Author information
Authors and Affiliations
Contributions
B.G. Sokolov and V.P. Boyarskiy: development of the concept and structure of the review, combination of the collected material, and final manuscript preparation; V.V. Norin and E.A. Sidel’nikova: collection of materials concerning promotion of cobalt carbonyls with Lewis bases; A.V. Kameshkov and E.A. Skadkovskaya: processing of materials on the development of supported cobalt catalysts promoted with phosphine ligands as applied to modern indistry.
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated from Zhurnal Prikladnoi Khimii, No. 5, pp. 563–578, May, 2022 https://doi.org/10.31857/S0044461822050026
Rights and permissions
About this article
Cite this article
Sokolov, B.G., Norin, V.V., Sidel’nikova, E.A. et al. Cobalt Catalysts for Hydroformylation and Carboalkoxylation: History and Commercial Prospects. Russ J Appl Chem 95, 631–645 (2022). https://doi.org/10.1134/S1070427222050020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427222050020