Abstract
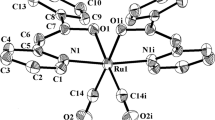
2+1 tricarbonyl complexes [Re(CO)3(N^N)CNCH2COOEt]X, where N^N = 2,2′-bipyridine (bipy) or 1,10-phenanthroline (phen) and X = Cl– or ClO4–, were prepared and isolated by two procedures. The intermediate tricarbonyl complexes [Re(CO)3(N^N)X] (where X = Cl– or ClO4–) were also isolated. The composition and molecular structure of these complexes were determined by single crystal X-ray diffraction analysis. The complexes have fac-tricarbonyl structure with bidentate coordination of bipy and phen. The complexes were characterized by PL, IR, UV-Vis, and 1H NMR spectroscopy and by elemental analysis.




Similar content being viewed by others
REFERENCES
Alberto, R., Top. Curr. Chem., 2005, vol. 252, p. 1. https://doi.org/10.1007/b101223
Arevalo, R., López, R., Falvello, L.R., Riera, L., and Perez, J., Chem. Eur. J., 2021, vol. 27, p. 379. https://doi.org/10.1002/chem.202003814
Garcia, R., Paulo, A., and Santos, I., Inorg. Chim. Acta, 2009, vol. 362, p. 4315. https://doi.org/10.1016/j.ica.2009.06.034
Agorastos, N., Borsig, L., Renard, A., Antoni, P., Giampietro Viola, G., Spingler, B., Kurz, P., and Alberto, R., Chem. Eur. J., 2007, vol. 13, p. 3842. https://doi.org/10.1002/chem.200700031
Silva, F., Fernandes, C., Campello, M.P.C., and Paulo, A., Polyhedron, 2017, vol. 125, p. 186. https://doi.org/10.1016/j.poly.2016.11.040
Chakraborty, I., Jimenez, J., Sameera, W.M.C., Kato, M., and Mascharak, P.K., Inorg. Chem., 2017, vol. 56, no. 5, p. 2863. https://doi.org/10.1021/acs.inorgchem.6b02999
Hostachy, S., Policar, C., and Delsuc, N., Coord. Chem. Rev., 2017, vol. 351, p. 172. https://doi.org/10.1016/J.CCR.2017.05.004
Murphy, B.L., Marker, S.C., Lambert, V.J., Woods, J.J., MacMillan, S.N., and Wilson, J.J., J. Organomet. Chem., 2020, vol. 907, article no. 121064. https://doi.org/10.1016/j.jorganchem.2019.121064
Miroslavov, A.E., Lumpov, A.A., Sidorenko, G.V., Levitskaya, E.M., Gorshkov, N.I., Suglobov, D.N., Alberto, R., Braband, H., Gurzhiy, V.V., Krivovichev, S.V., and Tananaev, I.G., J. Organomet. Chem., 2008, vol. 693, p. 4. https://doi.org/10.1016/j.jorganchem.2007.09.032
KomReddy, V., Ensz, K., Nguyen, H., Rillema, D.P., and Moore, C.E., J. Mol. Struct., 2021, vol. 1223, article no. 128739. https://doi.org/10.1016/j.molstruc.2020.128739
Marti, A.A., Mezei, G., Maldonado, L., Paralitici, G., Raptis, R.G., and Colon, J.L., Eur. J. Inorg. Chem., 2005, p. 118. https://doi.org/10.1002/ejic.200400531
Miroslavov, A.E., Gurziy, V.V., Tyupina, M.Yu., Lumpov, A.A., Sidorenko, G.V., Polotskii, Yu.S., and Suglobov, D.N., J. Organomet. Chem., 2013, vol. 745, p. 219. https://doi.org/10.1016/j.jorganchem.2013.07.019
Miroslavov, A.E., Sidorenko, G.V., Tyupina, M.Yu., and Gurzhiy, V.V., Russ. J. Gen. Chem., 2020, vol. 90, no. 12, p. 2333. https://doi.org/10.1134/S1070363220120178
Horn, E. and Snow, M.R., Aust. J. Chem., 1980, vol. 33, p. 2369. https://doi.org/10.1071/CH9802369
CrysAlisPro, Rigaku Oxford Diffraction, Version 1.171.39.35a, 2017.
Sheldrick, G.M., SADABS, Germany: Univ. Göttingen, 2007.
CrysAlisPro, Rigaku Oxford Diffraction, Version 2.171.39.35a, 2017.
Sheldrick, G.M., Acta Crystallogr., Sect. A, 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Sheldrick, G.M., Acta Crystallogr., Sect. C, 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053273314026370
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H., J. Appl. Crystallogr., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Funding
The study was financially supported by the Russian Foundation for Basic Research (project no. 19-33-90040\19).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary information
Rights and permissions
About this article
Cite this article
Tyupina, M.Y., Miroslavov, A.E., Sidorenko, G.V. et al. 2+1 Rhenium Tricarbonyl Complexes with N,N′-Bidentate Ligands and Ethyl Isocyanoacetate: Synthesis, Structure, and Properties. Russ J Gen Chem 92, 69–78 (2022). https://doi.org/10.1134/S1070363222010108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363222010108