Abstract
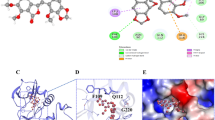
In order to discover novel and effective anti-tumor drugs, a series of new 2,4,6-trisubstituted quinazoline derivatives containing acrylamide structure have been designed, synthesized and evaluated for their antitumor activity against six human tumor cell lines (PC-3, Eca-109, MGC-803, HGC-27, A549 and H1975) by MTT assay, and compound N-(3-((6-methoxy-2-((3-methylbenzyl)thio)quinazolin-4-yl)amino)phenyl)acrylamide displayed the best antiproliferative activity against PC-3 cells (IC50 = 1.28 ± 0.63 μM), which was better than the positive control Gefitinib. Further mechanism research showed that this compound could significantly inhibited the migration and colony formation of PC-3 cells. At the same time, this compound induced PC-3 cells apoptosis through increasing the expression of pro-apoptotic proteins Bax and p53 and down-regulating the anti-apoptotic protein Bcl-2. This compound could also bound tightly to the active pocket of EGFR. Collectively, our findings suggested that this compound deserves further reseach as a potent antitumor agent for cancer therapy.







Similar content being viewed by others
REFERENCES
Ye, T., Han, Y., Wang, R., Yan, P., Chen, S., Hou, Y., and Zhao, Y., Bioorg. Chem., 2020, vol. 99, article no. 103796. https://doi.org/10.1016/j.bioorg.2020.103796
Hughes, D.J., Cook, G.J.R., McLean, E., Smith, D., King, J., Diamantopoulos, A., Jones, A., Neat, M., Santis, G., Spicer, J., Karapanagiotou, E., and Georgiou, A., Curr. Problems Cancer: Case Rep., 2021, vol. 4, article no. 100106. https://doi.org/10.1016/j.cpccr.2021.100106
Dilebo, K.B., Gumede, N.J., Nxumalo, W., Matsebatlela, T.M., Mangokoana, D., Moraone, N.R., Omondi, B., and Mampa, R.M., J. Mol. Struct., 2021, vol. 1243, article no. 130824. https://doi.org/10.1016/j.molstruc.2021.130824
Al-Salem, H.S., Hegazy, G.H., El-Taher, K.E., El-Messery, S.M., Al-Obaid, A.M., and El-Subbagh, H.I., Bioorg. Med. Chem. Lett., 2015, vol. 25, pp. 1490–1499. https://doi.org/10.1016/j.bmcl.2015.02.025
Dutta, A. and Sarma, D., Tuberculosis, 2020, vol. 124, article no. 101986. https://doi.org/10.1016/j.tube.2020.101986
Martynenko, Y., Antypenko, O., Nosulenko, I., Berest, G., and Kovalenko, S., Antiinflamm. Antiallergy Agents Med. Chem., 2020, vol. 19, pp. 61–73. https://doi.org/10.2174/1871523018666190115092215
Mujeeb Ur, R., Rathore, A., Siddiqui, A.A., Parveen, G., and Yar, M.S., J. Enzyme Inhib. Med. Chem., 2014, vol. 29, pp. 733–743. https://doi.org/10.3109/14756366.2013.845820
Modh, R.P., De Clercq, E., Pannecouque, C., and Chikhalia, K.H., J. Enzyme Inhib. Med. Chem., 2014, vol. 29, pp. 100–108. https://doi.org/10.3109/14756366.2012.755622
Argiris, A. and Mittal, N., Lung. Cancer, 2004, vol. 43, pp. 317–322. https://doi.org/10.1016/j.lungcan.2003.10.010
Imai, H., Kaira, K., Suzuki, K., Anzai, M., Tsuda, T., Ishizuka, T., Kuwako, T., Naruse, I., Nemoto, K., Uchino, J., Morozumi, N., Ishihara, S., Minato, K., and Hisada, T., Lung. Cancer, 2018, vol. 126, pp. 41–47. https://doi.org/10.1016/j.lungcan.2018.10.014
Kobayashi, Y., Fujino, T., Nishino, M., Koga, T., Chiba, M., Sesumi, Y., Ohara, S., Shimoji, M., Tomizawa, K., Takemoto, T., and Mitsudomi, T., J. Thorac. Oncol., 2018, vol. 13, pp. 727–731. https://doi.org/10.1016/j.jtho.2018.01.009
Gavriil, E.S., Doukatas, A., Karampelas, T., Myrianthopoulos, V., Dimitrakis, S., Mikros, E., Marakos, P., Tamvakopoulos, C., and Pouli, N., Eur. J. Med. Chem., 2019, vol. 176, pp. 393–409. https://doi.org/10.1016/j.ejmech.2019.05.029
Bansal, R. and Malhotra, A., Eur. J. Med. Chem., 2021, vol. 211, article no. 113016. https://doi.org/10.1016/j.ejmech.2020.113016
Shi, H., Lai, B., Chen, S., Zhou, X., Nie, J., and Ma, J.-A., Chin. J. Chem., 2017, vol. 35, pp. 1693–1700. https://doi.org/10.1002/cjoc.201700240
Cross, D.A.E., Ashton, S.E., Ghiorghiu, S., Eberlein, C., Nebhan, C.A., Spitzler, P.J., Orme, J.P., Finlay, M.R.V., Ward, R.A., Mellor, M.J., Hughes, G., Rahi, A., Jacobs, V.N., Brewer, M.R., Ichihara, E., Sun, J., Jin, H., Ballard, P., Al-Kadhimi, K., Rowlinson, R., Klinowska, T., Richmond, G.H.P., Cantarini, M., Kim, D.-W., Ranson, M.R., and Pao, W., Cancer Dis., 2014, vol. 4, pp. 1046–1061. https://doi.org/10.1158/2159-8290.Cd-14-0337
Walter, A.O., Sjin, R.T., Haringsma, H.J., Ohashi, K., Sun, J., Lee, K., Dubrovskiy, A., Labenski, M., Zhu, Z., Wang, Z., Sheets, M., St Martin, T., Karp, R., van Kalken, D., Chaturvedi, P., Niu, D., Nacht, M., Petter, R.C., Westlin, W., Lin, K., Jaw-Tsai, S., Raponi, M., Van Dyke, T., Etter, J., Weaver, Z., Pao, W., Singh, J., Simmons, A.D., Harding, T.C., and Allen, A., Cancer Dis., 2013, vol. 3, pp. 1404–1415. https://doi.org/10.1158/2159-8290.CD-13-0314
Zhou, W., Ercan, D., Chen, L., Yun, C.H., Li, D., Capelletti, M., Cortot, A.B., Chirieac, L., Iacob, R.E., Padera, R., Engen, J.R., Wong, K.K., Eck, M.J., Gray, N.S., and Jänne, P.A., Nature, 2009, vol. 462, pp. 1070–1074. https://doi.org/10.1038/nature08622
Chen, L., Chi, F., Wang, T., Wang, N., Li, W., Liu, K., Shu, X., Ma, X., and Xu, Y., Bioorg. Med. Chem., 2018, vol. 26, pp. 6087–6095. https://doi.org/10.1016/j.bmc.2018.11.009
Hao, Y., Lyu, J., Qu, R., Sun, D., Zhao, Z., Chen, Z., Ding, J., Xie, H., Xu, Y., and Li, H., Sci. Rep., 2017, vol. 7, article no. 3830. https://doi.org/10.1038/s41598-017-04184-9
Liu, Z., Wang, L., Feng, M., Yi, Y., Zhang, W., Liu, W., Li, L., Liu, Z., Li, Y., and Ma, X., Bioorg. Chem., 2018, vol. 77, pp. 593–599. https://doi.org/10.1016/j.bioorg.2018.01.035
Patel, H., Pawara, R., Ansari, A., and Surana, S., Eur. J. Med. Chem., 2017, vol. 142, pp. 32–47. https://doi.org/10.1016/j.ejmech.2017.05.027
Pawara, R., Ahmad, I., Nayak, D., Wagh, S., Wadkar, A., Ansari, A., Belamkar, S., Surana, S., Nath Kundu, C., Patil, C., and Patel, H., Bioorg. Chem., 2021, vol. 115, article no. 105234. https://doi.org/10.1016/j.bioorg.2021.105234
Wang, Z., Liu, L., Dai, H., Si, X., Zhang, L., Li, E., Yang, Z., Chao, G., Zheng, J., Ke, Y., Lihong, S., Zhang, Q., and Liu, H., Bioorg. Med. Chem., 2021, vol. 43, article no. 116265. https://doi.org/10.1016/j.bmc.2021.116265
Bhatia, P., Sharma, V., Alam, O., Manaithiya, A., Alam, P., Kahksha, Alam, M.T., and Imran, M., Eur. J. Med. Chem., 2020, vol. 204, article no. 112640. https://doi.org/10.1016/j.ejmech.2020.112640
He, J., Zhou, Z., Sun, X., Yang, Z., Zheng, P., Xu, S., and Zhu, W., Eur. J. Med. Chem., 2021, vol. 210, article no. 112995. https://doi.org/10.1016/j.ejmech.2020.112995
Reddy, B., Naidu, A., and Dubey, P.K., Asian J. Chem., 2013, vol. 25, pp. 2644–2646. https://doi.org/10.14233/ajchem.2013.13593
Song, P., Cui, F., Li, N., Xin, J., Ma, Q., Meng, X., Wang, C., Cao, Q., Gu, Y., Ke, Y., Zhang, Q., and Liu, H., Chin. J. Chem., 2017, vol. 35, pp. 1633–1639. https://doi.org/10.1002/cjoc.201700005
Wei, X.W., Yuan, J.M., Huang, W.Y., Chen, N.Y., Li, X.J., Pan, C.X., Mo, D.L., and Su, G.F., Eur. J. Med. Chem., 2020, vol. 186, article no. 111851. https://doi.org/10.1016/j.ejmech.2019.111851
Sun, B., Liu, X., Zheng, X., Wang, C., Meng, Q., Sun, H., Shu, X., Liu, K., Sun, X., Li, Y., and Ma, X., ChemMedChem, 2020, vol. 15, pp. 182–187. https://doi.org/10.1002/cmdc.201900606
Ge, Y., Yang, H., Wang, C., Meng, Q., Li, L., Sun, H., Zhen, Y., Liu, K., Li, Y., and Ma, X., Bioorg. Med. Chem., 2017, vol. 25, pp. 765–772. https://doi.org/10.1016/j.bmc.2016.11.054
Zhu, Y., Zheng, X., Wang, C., Sun, X., Sun, H., Ma, T., Li, Y., Liu, K., Chen, L., and Ma, X., Bioorg. Med. Chem., 2020, vol. 28, article no. 115254. https://doi.org/10.1016/j.bmc.2019.115254
Funding
Project supported by the National Natural Science Foundation of China (no. 82020108030) and this work was supported by National Key Research Program of Proteins (no. 2018YFE0195100) and Openning fund from State Key Laboratory of Esophageal Cancer Prevention & Treatment (no. K2020000X).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
The work has no studies involving humans or animals as subjects of the study.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Honglin, D., Chao, G., Xiaojie, S. et al. Design, Synthesis, and Antitumor Activity Evaluation of 2,4,6-Trisubstituted Quinazoline Derivatives Containing Acrylamide. Russ J Bioorg Chem 48, 1089–1100 (2022). https://doi.org/10.1134/S1068162022050090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022050090