Abstract
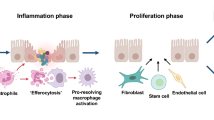
The regeneration of injured tissues is a complex physiological process. The immune system plays an indispensable role in the regeneration process and internal environment stability of damaged tissues. Heavy immune cell infiltration during wound healing stages of regeneration, aggregation of immune cells not only help to remove cell debris, but also secrete chemokines and cell growth factors. Among them, chemo-kines are the key factors in the process of regeneration, including Cys (C), Cys Cys (CC), Cys-x-Cys (CXC) and Cys-x3-Cys (CX3C) subfamilies, which are involved in regulating the migration and activation of immune cells, angiogenesis, functional changes of fibroblasts. This review focuses on the relationship among chemokines and immune cells and regenerative ability during limb regeneration, demonstrates the important contribution of chemokines in limb regeneration of salamander and explores the therapeutic potential of targeting chemokines as a new method to improve tissue regeneration.


Similar content being viewed by others
REFERENCES
Abnave, P. and Ghigo, E., Role of the immune system in regeneration and its dynamic interplay with adult stem cells, Semin. Cell Dev. Biol., 2019, vol. 87, pp. 160–168.
Alibardi, L. Review: limb regeneration in humans: dream or reality?, Ann. Anat., 2018, vol. 217, pp. 1–6.
Arslan, F., Houtgraaf, J.H., Keogh, B., et al., Treatment with OPN-305, a humanized anti-Toll-Like receptor-2 antibody, reduces myocardial ischemia/reperfusion injury in pigs, Circ. Cardiovasc. Interv., 2012, vol. 5, no. 2, pp. 279–287.
Balabanian, K., Lagane, B., Infantino, S., et al., The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes, J. Biol. Chem., 2005, vol. 280, no. 42, pp. 35760–35766.
Beddaoui, M., Coupland, S.G., and Tsilfidis, C., Recovery of function following regeneration of the damaged retina in the adult newt, Notophthalmus viridescens, Doc. Ophthalmol., 2012, vol. 125, no. 2, pp. 91–100.
Bolaños-Castro, L.A., Walters, H.E., García Vázquez, R.O., et al., Immunity in salamander regeneration: where are we standing and where are we headed?, Dev. Dyn., 2021, vol. 250, no. 6, pp. 753–767.
Bonecchi, R., Galliera, E., Borroni, E.M., et al., Chemokines and chemokine receptors: an overview, Front. Biosci. (Landmark Ed.), 2009, vol. 14, pp. 540–551.
Chen, G. and Robert, J., Antiviral immunity in amphibians, Viruses, 2011, vol. 3, no. 11, pp. 2065–2086.
Cheng, F., Shen, Y., Mohanasundaram, P., et al., Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling, Proc. Natl. Acad. Sci. U. S. A., 2016, vol. 113, no. 30, pp. E4320–E4327.
Choi, Y.H., Burdick, M.D., Strieter, B.A., et al., CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells, Mol. Cancer Res., 2014, vol. 12, no. 1, pp. 38–47.
Choi, J., Selmi, C., Leung, P.S., et al., Chemokine and chemokine receptors in autoimmunity: the case of primary biliary cholangitis, Expert Rev. Clin. Immunol., 2016, vol. 12, no. 6, pp. 661–672.
Christen, B., Robles, V., Raya, M., et al., Regeneration and reprogramming compared, BMC Biol., 2010, vol. 8, p. 5.
Clarke, C.N., Kuboki, S., Tevar, A., et al., CXC chemokines play a critical role in liver injury, recovery, and regeneration, Am. J. Surg., 2009, vol. 198, no. 3, pp. 415–419.
Cohen, N.J.A.Z., Amphibian Transplantation Reactions: A Review, Am. Zool., 1971, vol. 11, no. 2, pp. 193–205.
Costantini, C., Micheletti, A., Calzetti, F., et al., Neutrophil activation and survival are modulated by interaction with NK cells, Int. Immunol., 2010, vol. 22, no. 10, pp. 827–838.
Dreymueller, D., Denecke, B., Ludwig, A., et al., Embryonic stem cell-derived M2-like macrophages delay cutaneous wound healing, Wound Repair Regen., 2013, vol. 21, no. 1, pp. 44–54.
Feng, G., Hao, D., and Chai, J., Processing of CXCL12 impedes the recruitment of endothelial progenitor cells in diabetic wound healing, FEBS J., 2014, vol. 281, no. 22, pp. 5054–5062.
Frangogiannis, N.G., Matricellular proteins in cardiac adaptation and disease, Physiol. Rev., 2012, vol. 92, no. 2, pp. 635–688.
Freichel, M., Berlin, M., Schürger, A., et al., TRP channels in the heart, in Neurobiology of TRP Channels, Emir, T.L.R., Ed., Frontiers in Neuroscience, Boca Raton, FL: CRC Press/Taylor and Francis, 2017, pp. 149–185.
Geering, B., Stoeckle, C., Conus, S., et al., Living and dying for inflammation: neutrophils, eosinophils, basophils, Trends Immunol., 2013, vol. 34, no. 8, pp. 398–409.
Ghigo, A., Franco, I., Morello, F., et al., Myocyte signalling in leucocyte recruitment to the heart, Cardiovasc. Res., 2014, vol. 102, no. 2, pp. 270–280.
Ghosh, S., Thorogood, P., and Ferretti, P., Regenerative capability of upper and lower jaws in the newt, Int. J. Dev. Biol., 1994, vol. 38, no. 3, pp. 479–490.
Godwin, J.W. and Brockes, J.P., Regeneration, tissue injury and the immune response, J. Anat., 2006, vol. 209, no. 4, pp. 423–432.
Godwin, J.W. and Rosenthal, N., Scar-free wound healing and regeneration in amphibians: immunological influences on regenerative success, Differentiation, 2014, vol. 87, no. 1–2, pp. 66–75.
Godwin, J.W., Pinto, A.R., and Rosenthal, N.A., Macrophages are required for adult salamander limb regeneration, Proc. Natl. Acad. Sci. U. S. A., 2013, vol. 110, no. 23, pp. 9415–9420.
Godwin, J.W., Debuque, R., Salimova, E., et al., Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape, NPJ Regen. Med., 2017, vol. 2, pp. 1–11. https://doi.org/10.1038/s41536-017-0027-y
Goncharova, L.B. and Tarakanov, A.O., Why chemokines are cytokines while their receptors are not cytokine ones?, Curr. Med. Chem., 2008, vol. 15, no. 13, pp. 1297–1304.
Griffith, J.W., Sokol, C.L., and Luster, A.D., Chemokines and chemokine receptors: positioning cells for host defense and immunity, Annu. Rev. Immunol., 2014, vol. 32, pp. 659–702.
Grymula, K., Tarnowski, M., Wysoczynski, M., et al., Overlapping and distinct role of CXCR7-SDF-1/ITAC and CXCR4-SDF-1 axes in regulating metastatic behavior of human rhabdomyosarcomas, Int. J. Cancer, 2010, vol. 127, no. 11, pp. 2554–2568.
Guo, S. and Dipietro, L.A., Factors affecting wound healing, J. Dent. Res., 2010, vol. 89, no. 3, pp. 219–229.
Haas, B.J. and Whited, J.L., Advances in decoding axolotl limb regeneration, Trends Genet., 2017, vol. 33, no. 8, pp. 553–565.
Hardtke, S., Ohl, L., and Förster, R., Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help, Blood, 2005, vol. 106, no. 6, pp. 1924–1931.
Harty, M., Neff, A.W., King, M.W., et al., Regeneration or scarring: an immunologic perspective, Dev. Dyn., 2003, vol. 226, no. 2, pp. 268–279.
Heijink, I.H., Pouwels, S.D., Leijendekker, C., et al., Cigarette smoke-induced damage-associated molecular pattern release from necrotic neutrophils triggers proinflammatory mediator release, Am. J. Respir. Cell Mol. Biol., 2015, vol. 52, no. 5, pp. 554–562.
Heissig, B., Nishida, C., Tashiro, Y., et al., Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration, Histol. Histopathol., 2010, vol. 25, no. 6, pp. 765–770.
Henry, J.J. and Tsonis, P.A., Molecular and cellular aspects of amphibian lens regeneration, Prog. Retin. Eye Res., 2010, vol. 29, no. 6, pp. 543–555.
Herter, J. and Zarbock, A., Integrin regulation during leukocyte recruitment, J. Immunol., 2013, vol. 190, no. 9, pp. 4451–4457.
Janssens, R., Struyf, S., and Proost, P., The unique structural and functional features of CXCL12, Cell Mol. Immunol., 2018, vol. 15, no. 4, pp. 299–311.
Jewett, A., Tseng, H.C., Arasteh, A., et al., Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells, Curr. Drug Deliv., 2012, vol. 9, no. 1, pp. 5–16.
Kaufman, J., Ferrone, S., Flajnik, M., et al., MHC-like molecules in some nonmammalian vertebrates can be detected by some cross-reactive monoclonal antibodies, J. Immunol., 1990, vol. 144, no. 6, pp. 2273–2280.
Kaufman, J., Völk, H., and Wallny, H.J., A “minimal essential Mhc” and an “unrecognized Mhc”: two extremes in selection for polymorphism, Immunol. Rev., 1995, vol. 143, pp. 63–88.
Keeley, E.C., Mehrad, B., and Strieter, R.M., Chemokines as mediators of tumor angiogenesis and neovascularization, Exp. Cell Res., 2011, vol. 317, no. 5, pp. 685–690.
Keyes, K.T., Ye, Y., Lin, Y., et al., Resolvin E1 protects the rat heart against reperfusion injury, Am. J. Physiol. Heart Circ. Physiol., 2010, vol. 299, no. 1, pp. H153–H164.
Koniski, A.D. and Cohen, N., Reproducible proliferative responses of salamander (Ambystoma mexicanum) lymphocytes cultured with mitogens in serum-free medium, Dev. Comp. Immunol., 1992, vol. 16, no. 6, pp. 441–451.
Korbecki, J., Grochans, S., Gutowska, I., et al., CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands, Int. J. Mol. Sci., 2020, vol. 21, no. 20.
Laing, K.J. and Secombes, C.J., Chemokines, Dev. Comp. Immunol., 2004, vol. 28, no. 5, pp. 443–460.
Lee, J.S., Frevert, C.W., Wurfel, M.M., et al., Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo, J. Immunol., 2003, vol. 170, no. 10, pp. 5244–5251.
Lee, S.J., Namkoong, S., Kim, Y.M., et al., Fractalkine stimulates angiogenesis by activating the Raf-1/MEK/ERK- and PI3K/Akt/eNOS-dependent signal pathways, Am. J. Physiol. Heart Circ. Physiol., 2006, vol. 291, no. 6, pp. H2836–H2846.
Leigh, N.D., Dunlap, G.S., Johnson, K., et al., Transcriptomic landscape of the blastema niche in regenerating adult axolotl limbs at single-cell resolution, Nat. Commun., 2018, vol. 9, no. 1, p. 5153.
Lévesque, M., Gatien, S., Finnson, K., et al., Transforming growth factor: beta signaling is essential for limb regeneration in axolotls, PLoS One, 2007, vol. 2, no. 11, article ID e1227.
Li, L., Yan, B., Shi, Y.Q., et al., Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration, J. Biol. Chem., 2012, vol. 287, no. 30, pp. 25353–25360.
Li, M., Sun, X., Zhao, J., et al., CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization, Cell Mol. Immunol., 2020, vol. 17, no. 7, pp. 753–764.
Liekens, S., Schols, D., and Hatse, S., CXCL12–CXCR4 axis in angiogenesis, metastasis and stem cell mobilization, Curr. Pharm. Des., 2010, vol. 16, no. 35, pp. 3903–3920.
Liu, W., Liu, D., Zheng, J., et al., Annulus fibrosus cells express and utilize C-C chemokine receptor 5 (CCR5) for migration, Spine J., 2017, vol. 17, no. 5, pp. 720–726.
Lounsbury, N., Advances in CXCR7 modulators, Pharmaceuticals (Basel), 2020, vol. 13, no. 2.
Luker, K.E., Steele, J.M., Mihalko, L.A., et al., Constitutive and chemokine-dependent internalization and recycling of CXCR7 in breast cancer cells to degrade chemokine ligands, Oncogene, 2010, vol. 29, no. 32, pp. 4599–4610.
Mahdavian Delavary, B., van der Veer, W.M., van Egmond, M., et al., Macrophages in skin injury and repair, Immunobiology, 2011, vol. 216, no. 7, pp. 753–762.
Mandal, P.K., Biswas, S., Mandal, G., et al., CCL2 conditionally determines CCL22-dependent Th2-accumulation during TGF-β-induced breast cancer progression, Immunobiology, 2018, vol. 223, no. 2, pp. 151–161.
Mantovani, A., Sica, A., Sozzani, S., et al., The chemokine system in diverse forms of macrophage activation and polarization, Trends Immunol., 2004, vol. 25, no. 12, pp. 677–686.
McCusker, C., Bryant, S.V., and Gardiner, D.M., The axolotl limb blastema: cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods, Regeneration (Oxf.), 2015, vol. 2, no. 2, pp. 54–71.
McHedlishvili, L., Mazurov, V., Grassme, K.S., et al., Reconstitution of the central and peripheral nervous system during salamander tail regeneration, Proc. Natl. Acad. Sci. U. S. A., 2012, vol. 109, no. 34, pp. E2258–E2266.
Mescher, A.L. and Neff, A.W., Regenerative capacity and the developing immune system, Adv. Biochem. Eng. Biotechnol., 2005, vol. 93, pp. 39–66.
Murawala, P., Tanaka, E.M., and Currie, J.D., Regeneration: the ultimate example of wound healing, Semin. Cell Dev. Biol., 2012, vol. 23, no. 9, pp. 954–962.
Naumann, U., Cameroni, E., Pruenster, M., et al., CXCR7 functions as a scavenger for CXCL12 and CXCL11, PLoS One, 2010, vol. 5, no. 2, article ID e9175.
Neves, J., Zhu, J., Sousa-Victor, P., et al., Immune modulation by MANF promotes tissue repair and regenerative success in the retina, Science, 2016, vol. 353, no. 6294, article ID aaf3646.
Nibbs, R., Graham, G., and Rot, A. Chemokines on the move: control by the chemokine “interceptors” Duffy blood group antigen and D6, Semin. Immunol., 2003, vol. 15, no. 5, pp. 287–294.
Palmqvist, C., Wardlaw, A.J., and Bradding, P., Chemokines and their receptors as potential targets for the treatment of asthma, Br. J. Pharmacol., 2007, vol. 151, no. 6, pp. 725–736.
Palomino, D.C. and Marti, L.C., Chemokines and immunity, Einstein (Sao Paulo), 2015, vol. 13, no. 3, pp. 469–473.
Pan, T., Wang, X., Fa Ng, T., et al., The changes in skeletal muscle ultrasructure and MGF during and after exhaustive exercise in rat, Chin. J. Sports Med., 2012.
Park, J.E. and Barbul, A., Understanding the role of immune regulation in wound healing, Am. J. Surg., 2004, vol. 187, no. 5a, pp. 11s–16s.
Payzin-Dogru, D. and Whited, J.L. An integrative framework for salamander and mouse limb regeneration, Int. J. Dev. Biol., 2018, vol. 62, nos. 6–8, pp. 393–402.
Pizza, F. X., Peterson, J.M., Baas, J.H., et al., Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice, J. Physiol., 2005, vol. 562, part 3, pp. 899–913.
Prabhu, S.D. and Frangogiannis, N.G., The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis, Circ. Res., 2016, vol. 119, no. 1, pp. 91–112.
Proudfoot, A.E., Handel, T.M., Johnson, Z., et al., Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines, Proc. Natl. Acad. Sci. U. S. A., 2003, vol. 100, no. 4, pp. 1885–1890.
Purushothaman, S., Elewa, A., and Seifert, A.W., Fgf-signaling is compartmentalized within the mesenchyme and controls proliferation during salamander limb development, Elife, 2019, vol. 8.
Qi, M. and Xin, S., FGF signaling contributes to atherosclerosis by enhancing the inflammatory response in vascular smooth muscle cells, Mol. Med. Rep., 2019, vol. 20, no. 1, pp. 162–170.
Rajagopal, S., Kim, J., Ahn, S., et al., Beta-arrestin but not G protein-mediated signaling by the “decoy” receptor CXCR7, Proc. Natl. Acad. Sci. U. S. A., 2010, vol. 107, no. 2, pp. 628–632.
Ridiandries, A., Tan, J.T., and Bursill, C.A., The role of CC-chemokines in the regulation of angiogenesis, Int. J. Mol. Sci., 2016, vol. 17, no. 11.
Ridiandries, A., Bursill, C., and Tan, J., Broad-spectrum inhibition of the CC-chemokine class improves wound healing and wound angiogenesis, Int. J. Mol. Sci., 2017, vol. 18, no. 1.
Ridiandries, A., Tan, J.T.M., and Bursill, C.A., The role of chemokines in wound healing, Int. J. Mol. Sci., 2018, vol. 19, no. 10.
Riise, R.E., Bernson, E., Aurelius, J., et al., TLR-stimulated neutrophils instruct NK cells to trigger dendritic cell maturation and promote adaptive T cell responses, J. Immunol., 2015, vol. 195, no. 3, pp. 1121–1128.
Roberson, S. and Halpern, M.E., Convergence of signaling pathways underlying habenular formation and axonal outgrowth in zebrafish, Development, 2017, vol. 144, no. 14, pp. 2652–2662.
Rodero, M.P. and Khosrotehrani, K., Skin wound healing modulation by macrophages, Int. J. Clin. Exp. Pathol., 2010, vol. 3, no. 7, pp. 643–653.
Rodgers, A.K., Smith, J.J., and Voss, S.R., Identification of immune and non-immune cells in regenerating axolotl limbs by single-cell sequencing, Exp. Cell Res., 2020, vol. 394, no. 2, article ID 112149.
Romagnani, P., Lasagni, L., Annunziato, F., et al., CXC chemokines: the regulatory link between inflammation and angiogenesis, Trends Immunol., 2004, vol. 25, no. 4, pp. 201–209.
Ruffell, D., Mourkioti, F., Gambardella, A., et al., A CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair, Proc. Natl. Acad. Sci. U. S. A., 2009, vol. 106, no. 41, pp. 17475–17480.
Russo, R.C., Garcia, C.C., Teixeira, M.M., et al., The CXCL8/IL-8 chemokine family and its receptors in inflammatory diseases, Expert Rev. Clin. Immunol., 2014, vol. 10, no. 5, pp. 593–619.
Singer, A.J. and Clark, R.A., Cutaneous wound healing, N. Engl. J. Med., 1999, vol. 341, no. 10, pp. 738–746.
Singh, M.V., Swaminathan, P.D., Luczak, E.D., et al., MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction, J. Mol. Cell Cardiol., 2012, vol. 52, no. 5, pp. 1135–1144.
Singh, B.N., Weaver, C.V., Garry, M.G., et al., Hedgehog and Wnt signaling pathways regulate tail regeneration, Stem Cells Dev., 2018, vol. 27, no. 20, pp. 1426–1437.
Sommer, F., Torraca, V., and Meijer, A.H., Chemokine receptors and phagocyte biology in zebrafish, Front. Immunol., 2020, vol. 11, p. 325.
Stålman, A., Bring, D., and Ackermann, P.W., Chemokine expression of CCL2, CCL3, CCL5 and CXCL10 during early inflammatory tendon healing precedes nerve regeneration: an immunohistochemical study in the rat, Knee Surg. Sports Traumatol. Arthrosc., 2015, vol. 23, no. 9, pp. 2682–2689.
Sugimoto, M.A., Sousa, L.P., Pinho, V., et al., Resolution of inflammation: what controls its onset?, Front. Immunol., 2016, vol. 7, p. 160.
Tataroglu, O., Zhao, X., Busza, A., et al., Retraction notice to: calcium and SOL protease mediate temperature resetting of circadian clocks, Cell, 2017, vol. 171, no. 1, p. 256.
Teller, P. and White, T.K., The physiology of wound healing: injury through maturation, Surg. Clin. North Am., 2009, vol. 89, no. 3, pp. 599–610.
Toumi, H., F’Guyer, S., and Best, T.M., The role of neutrophils in injury and repair following muscle stretch, J. Anat., 2006, vol. 208, no. 4, pp. 459–470.
Tournefier, A., Laurens, V., Chapusot, C., et al., Structure of MHC class I and class II cDNAs and possible immunodeficiency linked to class II expression in the Mexican axolotl, Immunol. Rev., 1998, vol. 166, pp. 259–277.
Tsai, S.L., Baselga-Garriga, C., and Melton, D.A., Blastemal progenitors modulate immune signaling during early limb regeneration, Development, 2019, vol. 146, no. 1.
Vågesjö, E., Öhnstedt, E., Mortier, A., et al., Accelerated wound healing in mice by on-site production and delivery of CXCL12 by transformed lactic acid bacteria, Proc. Natl. Acad. Sci. U. S. A., 2018, vol. 115, no. 8, pp. 1895–1900.
Walker, S.E., Nottrodt, R., Maddalena, L., et al., Retinoid X receptor α downregulation is required for tail and caudal spinal cord regeneration in the adult newt, Neural. Regen. Res., 2018, vol. 13, no. 6, pp. 1036–1045.
Wang, J., Neutrophils in tissue injury and repair, Cell Tissue Res., 2018, vol. 371, no. 3, pp. 531–539.
Watts, A.O., Verkaar, F., van der Lee, M.M., et al., β-Arrestin recruitment and G protein signaling by the atypical human chemokine decoy receptor CCX-CKR, J. Biol. Chem., 2013, vol. 288, no. 10, pp. 7169–7181.
Wood, S., Jayaraman, V., Huelsmann, E.J., et al., Pro-inflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response, PLoS One, 2014, vol. 9, no. 3, article ID e91574.
Xue, M. and Jackson, C.J., Extracellular matrix reorganization during wound healing and its impact on abnormal scarring, Adv. Wound Care (New Rochelle), 2015, vol. 4, no. 3, pp. 119–136.
Yan, X., Anzai, A., Katsumata, Y., et al., Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction, J. Mol. Cell Cardiol., 2013, vol. 62, pp. 24–35.
Yang, Z., Sharma, A.K., Linden, J., et al., CD4+ T lymphocytes mediate acute pulmonary ischemia-reperfusion injury, J. Thorac. Cardiovasc. Surg., 2009, vol. 137, no. 3, pp. 695–702; discussion 702.
Zhang, M., Qiu, L., Zhang, Y., et al., CXCL12 enhances angiogenesis through CXCR7 activation in human umbilical vein endothelial cells, Sci. Rep., 2017, vol. 7, no. 1, p. 8289.
Zheng, H., Functions of chemokines and their receptors, Immunol. J., 2004.
Zhou, T., Li, N., Jin, Y., et al., Chemokine C–C motif ligand 33 is a key regulator of teleost fish barbel development, Proc. Natl. Acad. Sci. U. S. A., 2018, vol. 115, no. 22, pp. E5018–E5027.
Zhou, Z., Zeiter, S., Schmid, T., et al., Effect of the CCL5-releasing fibrin gel for intervertebral disc regeneration, Cartilage, 2020, vol. 11, no. 2, pp. 169–180.
Zlotnik, A. and Yoshie, O., Chemokines: a new classification system and their role in immunity, Immunity, 2000, vol. 12, no. 2, pp. 121–127.
Zlotnik, A., Burkhardt, A. M., and Homey, B., Homeostatic chemokine receptors and organ-specific metastasis, Nat. Rev. Immunol., 2011, vol. 11, no. 9, pp. 597–606.
Funding
This work was supported by the National Natural Science Foundation of P. R. China (no. 31670996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mengli Xu, Su, J., Yue, Z. et al. Inflammation and Limb Regeneration: The Role of the Chemokines. Russ J Dev Biol 53, 180–191 (2022). https://doi.org/10.1134/S1062360422030055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1062360422030055