Abstract
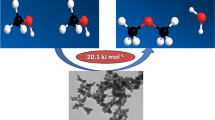
The effect of acid activation with 0.125–0.5 M Н2SO4, HCl, and HNO3 on the physicochemical properties and catalytic performance of natural clay (the Mukhartalinskii deposit) containing 95% montmorillonite (MM) was investigated in the synthesis of solketal [(2,2-dimethyl 1,3-dioxolan-4-yl)methanol] from glycerol and acetone. The reaction rate and selectivity toward solketal are shown to depend on the type and concentration of acid. Both the yield of solketal and the reaction rate rose with increasing acid concentration, which correlates with the increase in the number of Brønsted acid sites. The efficiency of the system was found to diminish in the order MM/HCl > MM/HNO3 > MM/H2SO4 as the surface acidity decreased.








Similar content being viewed by others
REFERENCES
Checa, M., Nogales-Delgado, S., Montes, V., and Encinar, J.M., Catalysts, 2020, vol. 10, no. 11. https://doi.org/10.3390/catal10111279
Bagnato, G., Iulianelli, A., Sanna, A., and Basile, A., Membranes, 2017, vol. 7, no. 2. https://doi.org/10.3390/membranes7020017
Nanda, M.R., Yuan, Z., Qin, W., and Xu, C., Catal. Rev., 2016, vol. 8, no. 3, pp. 309–336.
Maksimov, A.L., Nekhaev, A.I., and Ramazanov, D.N., Pet. Chem., 2015, vol. 55, no. 1, pp. 1–21. https://doi.org/10.1134/S0965544115010107
Correa, I., Faria, R.P.V., and Rodrigues, A.E., Sustainable Chem., 2021, vol. 2, pp. 286–324.
Mota, C.J.A., Silva, C.X.A., Rosenbach, N.J., Costa, J., and Silva, F., Energy Fuels, 2010, vol. 24, pp. 2733–2736.
US Patent 20090270643, 2009.
RF Patent 2365617, 2009.
Data Bridge Market Research. www.databridgemarketresearch.com/reports/global-solketal-market. Cited April 18, 2022.
Ferreira, P., Fonseca, I.M., Ramosa, M., Vital, J., and Castanheiro, J.E., Appl. Catal., B, 2010, vol. 98, nos. 1–2, pp. 94–99.
Nanda, M.R., Yuan, Z., Qin, W., Ghaziaskar, H.S., Poirier, M-A., and Xu, C., Appl. Energy, 2014, vol. 123, pp. 75–81.
Li, L., Korányi, T.I., Sels, B.F., and Pescarmona, P.P., Green Chem, 2012, vol. 14, no. 6, pp. 1611–1619.
Amri, S., Gómez, J., Balea, A., Merayo, N., Srasra, E., Besbes, N., and Ladero, M., Appl. Sci., 2019, vol. 9, no. 21, p. 4488. https://doi.org/10.3390/app9214488
Timofeeva, M.N., Panchenko, V.N., Krupskaya, V.V., Gil, A., and Vicente, M.A., Catal. Commun., 2017, vol. 90, pp. 65–69.
Ikonnikova, K.V., Ikonnikova, L.F., Minakova, T.S., and Sarkisov, Yu.S., Teoriya i praktika pH-metricheskogo opredeleniya kislotno-osnovnykh svoistv poverkhnosti tverdykh tel: uchebnoe posobie, (Theory and Practice of the pH-Metric Determination of Solids Surface Acid-Base Properties: a Textbook), Tomsk: Tomsk. Polytekhn. Univ., 2011.
Paukshtis, E.A., Infrakrasnaya spektroskopiya v geterogennom kislotnom-osnovnom katalize (Infrared Spectroscopy in Heterogeneous Acid-Base Catalysis), Novosibirsk: Nauka, 1992.
Krupskaya, V.V., Zakusin, S.V., Tyupina, E.A., Dorzhieva, O.V., Zhukhlistov, A.P., Belousov, P.E., and Timofeeva, M.N., Minerals, 2017, vol. 7, no. 4, pp. 49–64. https://doi.org/10.3390/min7040049
Farmer, V.C., in Data Handbook for Clay Materials and Other Non-Metallic Minerals, van Olphen, H. and Fripiat, J.J., Eds., Oxford, UK: Pergamon Press, 1979, pp. 285–337.
Angelini, M.M., Garrard, R.J., Rosen, S.J., and Hinrichs, R.Z., J. Phys. Chem. A, 2007, vol. 111, no. 17, pp. 3326–3335.
Flessnera, U., Jones, D.J., Rozière, J., Zajac, J., Storaro, L., Lenarda, M., Pavan, M., Jiménez-López, A., Rodríguez-Castellón, E., Trombetta, M., and Busca, G., J. Mol. Catal. A: Chem., 2001, vol. 168, pp. 247–256.
Tyagi, B., Chudasama, C.D., and Jasra, R.V., Spectrochim. Acta, Part A, 2006, vol. 64, pp. 273–278.
Krupskaya, V., Novikova, L., Tyupina, E., Belousova, P., Dorzhieva, O., Zakusin, S., Kimh, K., Roessneri, F., Badettij, E., Brunellij, A., and Belchinskay, L., Appl. Clay Sci., 2019, vol. 172, pp. 1–10.
Finevich, V.P., Allert, N.A., Karpova, T.R., and Duplyakin, V.K., Ross. Khim. Zh., 2007, vol. 51, no. 4, pp. 69–74.
Zatta, L., Ramos, L.P., and Wypych, F., Appl. Clay Sci., 2013, vols. 80–81, pp. 236–244.
Acid strength tables. http://primchem.narod.ru/chemistry/acids.html. Cited April 18, 2022.
Briones-Jurado, C. and Agacino-Valdés, E., J. Phys. Chem. A, 2009, vol. 113, no. 31, pp. 8994–9001. https://doi.org/10.1021/jp900236r
He, H., Guo, J., Xie, X., Lin, H., and Li, L., Clay Miner., 2002, vol. 37, no. 2, pp. 337–344. https://doi.org/10.1180/0009855023720037
Haffad, D., Chambellan, A., and Lavalley, J.C., Catal. Lett., 1998, vol. 54, pp. 227–233.
Jeon, I. and Nam, K., Sci. Rep., 2019, vol. 9. https://doi.org/10.1038/s41598-019-46175-y
Silva, C.X.A. and Mota, C.J.A., Biomass Bioenergy, 2011, vol. 35, no. 8, pp. 3547–3551.
Calvino-Casilda, V., Stawicka, K., Trejda, M., Ziolek, M., and Banares, M.A., J. Phys. Chem. C, 2014, vol. 118, pp. 10780–10791.
Marton, G.I., Iancu, P., Plesu, V., Marton, A., and Soriga, S.G., Rev. Chim. (Bucharest, Rom.), 2015, vol. 66, no. 5, pp. 750–753.
Ozorio, L.P., Pianzolli, R., Mota, M.B., and Mota, C.J.A., J. Braz. Chem. Soc., 2012, vol. 23, no. 5, pp. 931–937.
Pierpont, A.W., Batista, E.R., Martin, R.L., Chen, W., Kim, J.K., Hoyt, C.B., Gordon, J.C., Michalczyk, R., Silks, L.A.P., and Wu, R., ACS Catal., 2015, vol. 5, pp. 1013–1019.
US Patent 6890364, 2005.
EA Patent 018090, 2013.
ER Patent 2298851, 2014.
Funding
This work was supported by the RF Ministry of Higher Education and Science as part of a State Task for the Boreskov Institute of Catalysis, project no. AAAA-A21-121011390055-8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Kukharuk
Rights and permissions
About this article
Cite this article
Kovalenko, O.N., Simentsova, I.I., Panchenko, V.N. et al. Acid Activation of Montmorillonite as a Way of Controlling Its Catalytic Behavior in the Synthesis of Solketal from Glycerol and Acetone. Catal. Ind. 14, 208–217 (2022). https://doi.org/10.1134/S2070050422020040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070050422020040