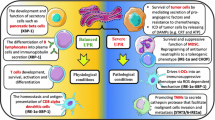
Abstract
Chemotherapy and radiotherapy are colossal stress factors for tumor cells. In response to therapy, the entire evolutionarily fixed response of cells to stress is activated. This happens at all levels of cell organization, namely, at the protein level and the DNA level. This response includes the cell proteostasis system, DNA-repair systems, tumor-suppressor genes, and many other cell systems. We will consider the role of the main systems of proteostasis in these processes, namely, macroautophagy and chaperones, which are parts of the integrated cell response to stress. As a result of the cell’s response to stress, the tumor cell becomes even less differentiated, activating the genes and intracellular systems necessary for survival. Cells that have responded to stress in this way have a more aggressive phenotype that is significantly more resistant to therapy. Under the influence of stress, the cell evolutionarily simplifies, which gives it additional chances for survival. Autophagy, on one hand, contributes to a decrease in tumor-cell differentiation and its plasticity, and, on the other hand, it maintains a certain stability, being responsible for the integrity of the genome and freeing the cell from damaged organelles and defective proteins. Both autophagy and chaperones contribute to the acquisition of multidrug resistance by the tumor, which further complicates therapy. Understanding these processes, taking into account the multistage nature of carcinogenesis, makes it possible to develop new therapeutic approaches.
Similar content being viewed by others
REFERENCES
Aggarwal, S., Tsuruo, T., and Gupta, S.J., Altered expression and function of P-glycoprotein (170 kDa), encoded by the MDR 1 gene, in T cell subsets from aging humans, Clin. Immunol., 1997, vol. 17, p. 448. https://doi.org/10.1023/a:1027363525408
Albakova, Z., Armeev, G.A., Kanevski, L.M., Kovalenko, E.I., and Sapozhnikov, A.M., HSP70 multi-functionality in cancer, Cells, 2020, vol. 9, p. 587. https://doi.org/10.3390/cells9030587
Anand, S.K., Sharma, A., Singh, N., and Kakkar, P., Entrenching role of cell cycle checkpoints and autophagy for maintenance of genomic integrity, DNA Repair (Amst.), 2020, vol. 86, p. 102748. https://doi.org/10.1016/j.dnarep.2019.102748
Apel, A., Herr, I., Schwarz, H., Rodemann, P., and Mayer, A., Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy, Cancer Res., 2008, vol. 68, p. 1485. https://doi.org/10.1158/0008-5472.CAN-07-0562
Benassi, B., Fanciulli, M., Fiorentino, F., Porrello, A., Chiorino, G., Loda, M., Zupi, G., and Biroccio, A., c-Myc phosphorylation is required for cellular response to oxidative stress, Mol. Cell, 2006, vol. 21, p. 509. https://doi.org/10.1016/j.molcel.2006.01.009
Bradley, E., Bieberich, E., Mivechi, N.F., Tangpisuthipongsa, D., and Wang, G., Regulation of embryonic stem cell pluripotency by heat shock protein 90, Stem Cells, 2012, vol. 30, p. 1624. https://doi.org/10.1002/stem.1143
Büchler, P., Reber, H.A., Lavey, R.S., Tomlinson, J., Büchler, M.W., Friess, H., and Hines, O.J., Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model, J. Surg. Res., 2004, vol. 120, p. 295. https://doi.org/10.1016/j.jss.2004.02.014
Chakraborty, C. and Agoramoorthy, G., Stem cells in the light of evolution, Indian J. Med. Res., 2012, vol. 135, p. 813.
Chao, T., Shih, H.T., Hsu, S.C., Chen, P.J., Fan, Y.S., Jeng, Y.M., Shen, Z.Q., Tsai, T.F., and Chang, Z.F., Autophagy restricts mitochondrial DNA damage-induced release of ENDOG (endonuclease G) to regulate genome stability, Autophagy, 2021, vol. 17, p. 3444. https://doi.org/10.1080/15548627.2021.1874209
Chen, N. and Karantza-Wadsworth, V., Role and regulation of autophagy in cancer, Biochim. Biophys. Acta, 2009, vol. 1793, p. 1516. https://doi.org/10.1016/j.bbamcr.2008.12.013
Condon, K.J. and Sabatini, D.M., Nutrient regulation of mTORC1 at a glance, J. Cell Sci., 2019, vol. 132, p. jcs222570. https://doi.org/10.1242/jcs.222570
Das, C.K., Mandal, M., and Kögel, D., Pro-survival autophagy and cancer cell resistance to therapy, Cancer Metastasis Rev., 2018a, vol. 37, p. 749. https://doi.org/10.1007/s10555-018-9727-z
Das, C.K., Linder, B., Bonn, F., Rothweiler, F., Dikic, I., Michaelis, M., Cinatl, J., Mandal, M., and Kögel, D., BAG3 overexpression and cytoprotective autophagy mediate apoptosis resistance in chemoresistant breast cancer cells, Neoplasia, 2018b, vol. 20, p. 263. https://doi.org/10.1016/j.neo.2018.01.001
Dinić, J., Podolski-Renić, A., Jovanović, M., Musso, L., Tsakovska, I., Pajeva, I., Dallavalle, S., and Pešić, M., Novel heat shock protein 90 inhibitors suppress P-glycoprotein activity and overcome multidrug resistance in cancer cells, Int. J. Mol. Sci., 2019, vol. 20, p. 4575. https://doi.org/10.3390/ijms20184575
Dubrez, L., Causse, S., Bonan, N.B., Dumétier, B., and Garrido, C., Heat-shock proteins: chaperoning DNA repair, Oncogene, 2020, vol. 39, p. 516. https://doi.org/10.1038/s41388-019-1016-y
Erenpreisa, J., Salmina, K., Anatskaya, O., and Cragg, M.S., Paradoxes of cancer: survival at the brink, Semin. Cancer Biol., 2022, vol. 81, p. 119. https://doi.org/10.1016/j.semcancer.2020.12.009
Feng, Y. and Klionsky, D.J., Autophagy regulates DNA repair through SQSTM1/p62, Autophagy, 2017, vol. 13, p. 995. https://doi.org/10.1080/15548627.2017.1317427
Galati, S., Boni, C., Gerra, M.C., Lazzaretti, M., and Buschini, A., Autophagy: a player in response to oxidative stress and DNA damage, Oxid. Med. Cell Longev., 2019, vol. 2019, p. 5692958. https://doi.org/10.1155/2019/5692958
Gomes, L.R., Menck, C.F.M., and Leandro, G.S., Autophagy roles in the modulation of DNA repair pathways, Int. J. Mol. Sci., 2017, vol. 18, p. 2351. https://doi.org/10.3390/ijms18112351
Gremke, N., Polo, P., Dort, A., Schneikert, J., Elmshäuser, S., Brehm, C., Klingmüller, U., Schmitt, A., Reinhardt, H.C., Timofeev, O., Wanzel, M., and Stiewe, T., mTOR-mediated cancer drug resistance suppresses autophagy and generates a druggable metabolic vulnerability, Nat. Commun., 2020, vol. 11, p. 4684. https://doi.org/10.1038/s41467-020-18504-7
Hewitt, G. and Korolchuk, V.I., Repair, reuse, recycle: the expanding role of autophagy in genome maintenance, Trends Cell Biol., 2017, vol. 27, p. 340. https://doi.org/10.1016/j.tcb.2016.11.011
Ikwegbue, P.C., Masamba, P., Mbatha, L.S., Oyinloye, B.E., and Kappo, A.P., Interplay between heat shock proteins, inflammation and cancer: a potential cancer therapeutic target, Am. J. Cancer Res., 2019, vol. 9, p. 242.
Jewer, M., Lee, L., Leibovitch, M., Zhang, G., Liu, J., Findlay, S.D., Vincent, K.M., Tandoc, K., Dieters-Castator, D., Quail, D.F., Dutta, I., Coatham, M., Xu, Z., Puri, A., Guan, B.J., et al., Translational control of breast cancer plasticity, Nat. Commun., 2020, vol. 11, p. 2498. https://doi.org/10.1038/s41467-020-16352-z
Juretschke, T. and Beli, P., Causes and consequences of DNA damage-induced autophagy, Matrix Biol., 2021, vols. 100–101, p. 39. https://doi.org/10.1016/j.matbio.2021.02.004
Kametaka, S., Okano, T., Ohsumi, M., and Ohsumi, Y., Apg14p and Apg6/Vps30p form a protein complex essential for autophagy in the yeast, Saccharomyces cerevisiae, J. Biol. Chem., 1998, vol. 273, p. 22284. https://doi.org/10.1074/jbc.273.35.22284
Karabicici, M., Alptekin, S., Fırtına Karagonlar, Z., and Erdal, E., Doxorubicin-induced senescence promotes stemness and tumorigenicity in EpCAM-/CD133-nonstem cell population in hepatocellular carcinoma cell line, HuH-7, Mol. Oncol., 2021, vol. 15, p. 2185. https://doi.org/10.1002/1878-0261.12916
Kim, H.B., Lee, S.H., Um, J.H., Oh, W.K., Kim, D.W., Kang, C.D., and Kim, S.H., Sensitization of multidrug-resistant human cancer cells to Hsp90 inhibitors by down-regulation of SIRT1, Oncotarget, 2015a, vol. 6, p. 36202. https://doi.org/10.18632/oncotarget.5343
Kim, B.M., Hong, Y., Lee, S., Liu, P., Lim, J.H., Lee, Y.H., Lee, T.H., Chang, K.T., and Hong, Y., Therapeutic implications for overcoming radiation resistance in cancer therapy, Int. J. Mol. Sci., 2015b, vol. 16, p. 26880. https://doi.org/10.3390/ijms161125991
Kögel, D., Linder, B., Brunschweiger, A., Chines, S., and Behl, C., At the crossroads of apoptosis and autophagy: multiple roles of the co-chaperone BAG3 in stress and therapy resistance of cancer, Cells, vol. 9, p. 574. https://doi.org/10.3390/cells9030574
Kumar, S., Stokes, J., 3rd, Singh, U.P., Scissum Gunn, K., Acharya, A., Manne, U., and Mishra, M., Targeting Hsp70: a possible therapy for cancer, Cancer Lett., 2016, vol. 374, p. 156. https://doi.org/10.1016/j.canlet.2016.01.056
Li, Y.J., Lei, Y.H., Yao, N., Wang, C.R., Hu, N., Ye, W.C., Zhang, D.M., Chen, Z.S., and Chin, J., Autophagy and multidrug resistance in cancer, Cancer, 2017, vol. 36, p. 52. https://doi.org/10.1186/s40880-017-0219-2
Liang, B., Liu, X., Liu, Y., Kong, D., Liu, X., Zhong, R., and Ma, S., Inhibition of autophagy sensitizes MDR-phenotype ovarian cancer SKVCR cells to chemotherapy, Biomed. Pharmacother., 2016, vol. 82, p. 98. https://doi.org/10.1016/j.biopha.2016.04.054
Lin, F., Gao, L., Su, Z., Cao, X., Zhan, Y., Li, Y., and Zhang, B., Knockdown of KPNA2 inhibits autophagy in oral squamous cell carcinoma cell lines by blocking p53 nuclear translocation, Oncol. Rep., 2018, vol. 40, p. 179. https://doi.org/10.3892/or.2018.6451
Liu, J., Chang, B., Li, Q., Xu, L., Liu, X., Wang, G., Wang, Z., and Wang, L., Redox-responsive dual drug delivery nanosystem suppresses cancer repopulation by abrogating doxorubicin-promoted cancer stemness, metastasis, and drug resistance, Adv. Sci. (Weinh.), 2019, vol. 6, p. 1801987. https://doi.org/10.1002/advs.201801987
Margulis, B., Tsimokha, A., Zubova, S., and Guzhova, I., Molecular chaperones and proteolytic machineries regulate protein homeostasis in aging cells, Cells, 2020, vol. 9, p. 1308. https://doi.org/10.3390/cells9051308
Mehta, A.P., Supekova, L., Chen, J.H., Petonjamasp, K., Webster, P., Ko, Y., Henderson, S.C., McDermott, G., Supek, F., and Schullz, P.G., Engineering yeast endosymbionts as a step toward the evolution of mitochondria, Proc. Natl. Acad. Sci. U. S. A., 2018, vol. 115, p. 11796–11801. https://doi.org/10.1073/pnas.1813143115
Mendez, F., Sandigursky, M., Franklin, W.A., Kenny, M.K., Kureekattil, R., and Bases, R., Heat-shock proteins associated with base excision repair enzymes in HeLa cells, Radiat. Res., 2000, vol. 153, pp. 186–195. https://doi.org/10.1667/0033-7587(2000)153[0186:hspawb]2.0.co;2
Menendez, J.A., Vellon, L., Oliveras-Ferraros, C., Cufí, S., and Vazquez-Martin, A., mTOR-regulated senescence and autophagy during reprogramming of somatic cells to pluripotency: a roadmap from energy metabolism to stem cell renewal and aging, Cell Cycle, 2011, vol. 10, p. 3658. https://doi.org/10.4161/cc.10.21.18128
Mizushima, N., Autophagy: process and function, Genes Dev., 2007, vol. 21, p. 2861. https://doi.org/10.1101/gad.1599207
Ozates, N.P., Soğutlu, F., Lerminoglu, F., Demir, B., Gunduz, C., and Shademan, B., Effects of rapamycin and AZD3463 combination on apoptosis, autophagy, and cell cycle for resistance control in breast cancer, Life Sci., 2021, vol. 264, p. 118643. https://doi.org/10.1016/j.lfs.2020.118643
Pan, Y., Gao, Y., Chen, L., Gao, G., Dong, H., Yang, Y., Dong, B., and Chen, X., Targeting autophagy augments in vitro and in vivo antimyeloma activity of DNA-damaging chemotherapy, Clin. Cancer Res., 2011, vol. 17, p. 3248. https://doi.org/10.1158/1078-0432.CCR-10-0890
Pandita, T.K., Higashikubo, R., and Hunt, C.R., HSP70 and genomic stability, Cell Cycle, 2004, vol. 3, p. 591. https://doi.org/10.4161/cc.3.5.863
Pani, G., Galeotti, T., and Chiarugi, P., Metastasis: cancer cell’s escape from oxidative stress, Cancer Metastasis Rev., 2010, vol. 29, p. 351. https://doi.org/10.1007/s10555-010-9225-4
Pennisi, R., Ascenzi, P., and di Masi, A., Hsp90: a new player in DNA repair?, Biomolecules, 2015, vol. 5, p. 2589. https://doi.org/10.3390/biom5042589
Quanz, M., Herbette, A., Sayarath, M., Koning, L., Dubois, T., Sun, J.S., and Dutreix, M., Heat shock protein 90α (Hsp90α) is phosphorylated in response to DNA damage and accumulates in repair foci, J. Biol. Chem., 2012, vol. 287, p. 8803. https://doi.org/10.1074/jbc.M111.320887
Roshani-Asl, E., Mansori, B., Mohammadi, A., Najafi, S., Danesh-Pouya, F., and Rasmi, Y., Interaction between DNA damage response and autophagy in colorectal cancer, Gene, 2020, vol. 730, p. 144323. https://doi.org/10.1016/j.gene.2019.144323
Roufayel, R. and Kadry, S., Molecular chaperone HSP70 and key regulators of apoptosis—a review, Curr. Mol. Med., 2019, vol. 19, p. 315. https://doi.org/10.2174/1566524019666190326114720
Sannino, S., Yates, M.E., Schurdak, M.E., Oesterreich, S., Lee, A.V., Wipf, P., and Brodsky, J.L., Unique integrated stress response sensors regulate cancer cell susceptibility when Hsp70 activity is compromised, Elife, 2021, vol. 10, p. e64977. https://doi.org/10.7554/eLife.64977
Smith, A.G. and Macleod, K.F., Autophagy, cancer stem cells and drug resistance, J. Pathol., 2019, vol. 7, p. 708. https://doi.org/10.1002/path.5222
Song, X., Lee, D.H., Dilly, A.K., Lee, Y.S., Choudry, H.A., Kwon, Y.T., Bartlett, D.L., and Lee, Y.J., Crosstalk between apoptosis and autophagy is regulated by the arginylated BiP/Beclin-1/p62 complex, Mol. Cancer Res., 2018, vol. 16, p. 1077. https://doi.org/10.1158/1541-7786.MCR-17-0685
Sottile, M.L. and Nadin, S.B., Heat shock proteins and DNA repair mechanisms: an updated overview, Cell Stress Chaperones, 2018, vol. 23, p. 303. https://doi.org/10.1007/s12192-017-0843-4
Stagni, V., Ferri, A., Cirotti, C., and Barilà, D., ATM kinase-dependent regulation of autophagy: a key player in senescence?, Front. Cell Dev. Biol., 2021, vol. 8, p. 599048. https://doi.org/10.3389/fcell.2020.599048
Sun, W.L., Lan, D., Gan, T.Q., and Cai, Z.W., Autophagy facilitates multidrug resistance development through inhibition of apoptosis in breast cancer cells, Neoplasma, 2015, vol. 62, p. 199. https://doi.org/10.4149/neo_2015_025
Tabata, M., Tsubaki, M., Takeda, T., Tateishi, K., Maekawa, S., Tsurushima, K., Imano, M., Satou, T., Ishizaka, T., and Nishida, S., Inhibition of HSP90 overcomes melphalan resistance through downregulation of Src in multiple myeloma cells, Clin. Exp. Med., 2020, vol. 20, p. 63. https://doi.org/10.1007/s10238-019-00587-2
Tian, Z.C., Wang, J.Q., and Ge, H.J., Apatinib ameliorates doxorubicin-induced migration and cancer stemness of osteosarcoma cells by inhibiting Sox2 via STAT3 signalling, Orthop. Translat., 2019, vol. 22, p. 132. https://doi.org/10.1016/j.jot.2019.07.003
Tian, X., Zhang, S., Zhou, L., Seyhan, A.A., Hernandez Borrero, L., Zhang, Y., and El-Deiry, W.S., Targeting the integrated stress response in cancer therapy, Front. Pharmacol., 2021, vol. 12, p. 747837. https://doi.org/10.3389/fphar.2021.747837
Trigos, A.S, Pearson, R.B, Papenfuss, A.T, and Goode, D.L., Altered interactions between unicellular and multicellular genes drive hallmarks of transformation in a diverse range of solid tumors, Proc. Natl. Acad. Sci. U. S. A., 2017, vol. 114, p. 6406.
Vilaboa, N.E., Galán, A., Troyano, A., and E de Blas Aller, P., Regulation of multidrug resistance 1 (MDR1)/P-glycoprotein gene expression and activity by heat-shock transcription factor 1 (HSF1), J. Biol. Chem., 2000, vol. 275, p. 24970. https://doi.org/10.1074/jbc.M909136199
Vilas-Boas, V., Silva, R., Gaio, A.R., Martins, A.M., Lima, S.C., Cordeiro-da-Silva, A., Bastos, M.L., and Remião, F., P-glycoprotein activity in human Caucasian male lymphocytes does not follow its increased expression during aging, Cytometry A, 2011, vol. 79, p. 912. https://doi.org/10.1002/cyto.a.21135
Wang, R., Shao, F., Liu, Z., Zhang, J., Wang, S., Liu, J., Liu, H., Chen, H., Liu, K., Xia, M., and Wang, Y., The Hsp90 inhibitor SNX-2112, induces apoptosis in multidrug resistant K562/ADR cells through suppression of Akt/NF-κB and disruption of mitochondria-dependent pathways, Chem. Biol. Interact., 2013, vol. 205, p. 1. https://doi.org/10.1016/j.cbi.2013.06.007
Wang, Y., Zhang, N., Zhang, L., Li, R., Fu, W., Ma, K., Li, X., Wang, L., Wang, J., Zhang, H., Gu, W., Zhu, W.G., and Zhao, Y., Autophagy regulates chromatin ubiquitination in DNA damage response through elimination of SQSTM1/p62, Mol. Cell, 2016, vol. 63, p. 34. https://doi.org/10.1016/j.molcel.2016.05.027
Wang, F., Xia, X., Yang, C., Shen, J., Mai, J., Kim, H.C., Kirui, D., Kang, Y., Fleming, J.B., Koay, E.J., Mitra, S., Ferrari, M., and Shen, H., SMAD4 gene mutation renders pancreatic cancer resistance to radiotherapy through promotion of autophagy, Clin. Cancer Res., 2018, vol. 24, p. 3176. https://doi.org/10.1158/1078-0432.CCR-17-3435
Wawrzynow, B., Zylicz, A., and Zylicz, M., Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action, Biochim. Biophys. Acta Rev. Cancer, 2018, vol. 1869, p. 161. https://doi.org/10.1016/j.bbcan.2017.12.004
Yang, S., Wang, X., Contino, G., Liesa, M., Sahin, E., Ying, H., Bause, A., Li, Y., Stommel, J.M., Dell’anto-nio, G., Mautner, J., Tonon, G., Haigis, M., Shiri-hai, O.S., Doglioni, C., et al., Pancreatic cancers require autophagy for tumor growth. Genes Dev., 2011, vol. 25, p. 717. https://doi.org/10.1101/gad.2016111
Zhang, H., Chen, J., Zeng, Z., Que, W., and Zhou, L., Knockdown of DEPTOR induces apoptosis, increases chemosensitivity to doxorubicin and suppresses autophagy in RPMI-8226 human multiple myeloma cells in vitro, Int. J. Mol. Med., 2013, vol. 31, p. 1127. https://doi.org/10.3892/ijmm.2013.1299
Zhang, D., Tang, B., Xie, X., Xiao, Y.F., Yang, S.M., and Zhang, J.W., The interplay between DNA repair and autophagy in cancer therapy, Cancer Biol. Ther., 2015, vol. 16, p. 1005. https://doi.org/10.1080/15384047.2015.1046022
Funding
This work was carried out with the financial support of the Russian Science Foundation, project no. 22-25-20229 (https://rscf.ru/project/22-25-20229/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This work did not include experiments involving animals or human beings.
Rights and permissions
About this article
Cite this article
Zubova, S.G., Gnedina, O.O. The Role of the Integrated Response of Tumor Cells to Stress, Autophagy, and Chaperones in the Origin of Recurrent Resistant Tumors. Cell Tiss. Biol. 17, 465–476 (2023). https://doi.org/10.1134/S1990519X23050139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X23050139