Abstract
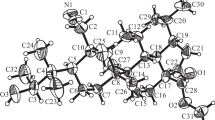
tert-Butyl 4-[(E)-but-1-en-3-yn-1-yl]-3-{[tert-butyl(dimethyl)silyl]oxy}-1H-indole-1-carboxylate has been synthesized with a good yield and selectivity starting from commercially available 4-bromo-1H-indole and using simple reagents. The title compound is a potential precursor to biologically active natural products like Indiacen A and Indiacen B. The newly synthesized compounds were characterized by spectral data.


Similar content being viewed by others
REFERENCES
Nguyen, T.T., Koh, M.H., Mann, T.J., Schrock, R.R., and Hoveyda, A.H., Nature, 2017, vol. 552, p. 347. https://doi.org/10.1038/nature25002
Marsch, N., Kock, M., and Lindel, T., Beilstein J. Org. Chem., 2016, vol. 12, p. 334. https://doi.org/10.3762/bjoc.12.36
Steinmetz, H., Mohr, K.I., Zander, W., Jansen, R., Gerth, K., and Müller, R., J. Nat. Prod., 2012, vol. 75, p. 1803. https://doi.org/10.1021/np300288b
Anantoju, K.K., Mohd, B.S., and Maringanti, T.C., Tetrahedron Lett., 2017, vol. 58, no. 15, p. 1499. https://doi.org/10.1016/j.tetlet.2017.03.002
Shaikh, T.M.A. and Debebe, H., J. Chem., 2020, vol. 9, article ID 4358453. https://doi.org/10.1155/2020/4358453
Adam, J.M., Cairns, J., Caulfield, W., Cowley, P., Cumming, I., Easson, M., Edwards, D., Ferguson, M., Goodwin, R., Jeremiah, F., Kiyoi, T., Mistry, A., Moir, E., Morphy, R., Tierney, J., York, M., Baker, J., Cottney, J., Houghton, A., Westwood, P., and Walker, G., MedChemComm, 2010, vol. 1, p. 54. https://doi.org/10.1039/C0MD00022A
Choppara, P., Prasad, Y.V., Rao, C.V., Hari Krishna, K., Trimoorthulu, G., Maheswara Rao, G.U., Venkateswara Rao, J., Bethu, M.S, and Murthy, Y.L.N., Arab. J. Chem., 2019, vol. 12, no. 8, p. 2328. https://doi.org/10.1016/j.arabjc.2015.02.006
Lauchli, R. and Shea, K.J., Org. Lett., 2006, vol. 8, p. 5287. https://doi.org/10.1021/ol0620747
Madadi, N.R., Penthala, N.R., Janganati, V., and Crooks, P.A., Bioorg. Med. Chem. Lett., 2014, vol. 24, p. 601. https://doi.org/10.1016/j.bmcl.2013.12.013
Singh, P., Mittal, A., Bhardwaj, A., Kaur, S., and Kumar, S., Bioorg. Med. Chem. Lett., 2008, vol. 18, p. 85. https://doi.org/10.1016/j.bmcl.2007.11.010
Mashayekhi, V., Tehrani, K.H.M.E., Azerang, P., Sardari, S., and Kobarfard, F., Arch. Pharmacal Res., 2021, vol. 44. p. 1. https://doi.org/10.1007/s12272-013-0242-z
Hall, A., Billinton, A., Brown, S.H., Chowdhury, A., Giblin, G.M.P., Goldsmith, P., Hurst, D.N., Naylor, A., Patel, S., and Scoccitti, T., Bioorg. Med. Chem. Lett., 2008, vol. 18. p. 2684. https://doi.org/10.1016/j.bmcl.2008.03.018
Moir, E.M., Yoshiizumi, K., Cairns, J., Cowley, P., Ferguson, M., Jeremiah, F., Kiyoi, T., Morphy, R., Tierney, J., Wishart, G., York, M., Baker, J., Cottney, J.E., Houghton, A.K., McPhail, P., Osprey, A., Walker, G., and Adam, J.A., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 7327. https://doi.org/10.1016/j.bmcl.2010.10.061
Madadi, N.R., Penthala, N.R., Brents, L.K., Ford, B.M., Prather, P.L., and Crooks, P.A., Bioorg. Med. Chem. Lett., 2013, vol. 23, p. 2019. https://doi.org/10.1016/j.bmcl.2013.02.025
Ryoichi, K. and Manabu, K., Org. Lett., 2006, vol. 8, no. 12, p. 2653. https://doi.org/10.1021/ol061039x
Badenock, J.C., Jordan, J.A., and Gribble, G.W., Tetrahedron Lett., 2013, vol. 54, nol. 22, p. 2759. https://doi.org/10.1016/j.tetlet.2013.02.116
Moyer, M.P., Shiurba, J.F., and Rapoport, H., J. Org. Chem., 1986, vol. 51, p. 5106. https://doi.org/10.1021/jo00376a010
Netz, N. and Opatz, T., J. Org. Chem., 2016, vol. 81, p. 1723. https://doi.org/10.1021/acs.joc.5b02815
Gibson, A.W., Humphrey, G.R., Kennedy, D.J., and Wright, S.H.B., Synthesis, 1991, vol. 1991, no. 5, p. 414. https://doi.org/10.1055/s-1991-26481
Burke, L.T., Dixon, D.J., Ley, S.V., and Rodríguez, F., Org. Lett., 2000, vol. 2, p. 3611. https://doi.org/10.1021/ol006493u
Wang, Y., Huang, B., Bin, H., Sheng, Sh., and Cai, M., J. Chem Res., 2007, no. 12, p. 728. https://doi.org/10.3184/030823407X275928
ACKNOWLEDGMENTS
The authors are thankful to Management and Principal, Sreenidhi Institute of Science and Technology, Hyderabad, India for providing necessary facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, B.R., Kumar, A.K., Reddy, B.S. et al. Synergic Synthesis and Characterization of tert-Butyl 4-[(E)-But-1-en-3-yn-1-yl]-3-{[tert-butyl(dimethyl)silyl]oxy}-1H-indole-1-carboxylate. Russ J Org Chem 58, 580–583 (2022). https://doi.org/10.1134/S1070428022040169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428022040169