Abstract
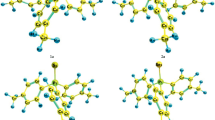
In keeping with the calculations of thermodynamic characteristics of possible transformations of tris(hydroxymethyl)phosphine in the framework of DFT-approach using hybrid exchange-correlation functional B3LYP with the basis 6–311++G** the probability of transformation of tris(hydroxymethyl)phosphine at the temperature below 350 K into methylbis(hydroxymethyl)phosphine oxide is higher than the conversion into methyl(hydroxymethyl)phosphine oxide, and at the temperature over 350 K the trend is opposite. The probability of the formation of a heterocyclic dimer at the temperature over 300 K is somewhat lower and of bis(hydroxymethyl)phosphine in the temperature range 250–550 K is significantly lower.
Similar content being viewed by others
References
Reuter, M., and Orthner, L., FRG Patent Appl. no. 1035135, 1959; Chem. Abstr., 1960, vol. 54, p. 14125.
Erastov, O.A. and Nikonov, G.N., Funktsional’nozameshchennye fosfiny i ikh proizvodnye (Functionally Substituted Phosphines and Their Derivatives), Moscow: Nauka, 1986.
Valetdinov, R.K., Goruchest’ polimernykh materialov. Mezhvuzovskii sbornik nauchykh trudov, Volgograd: izd. Volgogr. Polikhn. Inst., 1987, p. 43.
Petrov, K.A., and Parshina, V.A., Russ. Chem. Rev., 1968, vol. 37, p. 1218.
James, R., and Lorenzini, F., Coord. Chem. Rev., 2010, vol. 254, p. 420.
Marzano, C., Gandin, V., Pellei, M., Colavito, D., Papini, G., Lobbia, G.G., Del Giudice, E., Porchia, M., Tisato, F., and Santini, C., J. Med. Chem., 2008, vol. 51, p. 798.
Grekov, L.I., and Novakov, I.A., Kinet. Catal., 2006, vol. 47, p. 358.
Grekov, L.I., and Litinskii, A.O., Russ. J. Gen. Chem., 2009, vol. 79, p. 905.
Grekov, L.I., Kalinkin, D.P., and Litinskii, A.O., Russ. J. Gen. Chem., 2011, vol. 81, p. 1111.
Grekov, L.I., Vladimtseva, I.V., Efremenko, V.I., and Chernov, A.B., Biotecknologia, 2007, vol. 4, p. 34.
Trippett, S., J. Chem. Soc., 1961, p. 2813.
Buckler, Sh.A., J. Am. Chem. Soc., 1960, vol. 82, p. 4215.
Hellmann, H., Bader, J., Burkner, H., and Schumacher, O., Lieb. Ann., 1962, vol. 659, p. 49.
Valetdinov, R.K., Zuikova, A.N., Zyablikova, T.A., and Il’yasov, A.V., Zh. Obshch. Khim., 1979, vol. 49, p. 503.
Mukhutdinov, E.A., Mukhutdinov, A.A., Kovalenko, V.I., and Sol’yashinova, O.A., Russ. J. Phys. Chem. A, 2007, vol. 81, p. 747.
Minkin, V.I., Simkin, B.Ya., and Minyaev, R.M., Teoriya stroeniya molekul (Theory of Molecular Structure), Rostov-on-Don: Feniks, 1997.
Naumov, V.A. and Vilkov, L.V., Molekulyarnye struktury fosfororganicheskikh soedinenii (Molecular Structures of Organophosphorus Compounds), Moscow: Nauka, 1986.
Koch, W., A Chemist’ Guide to Density Functional Theory, Wiley-VCH, 2001.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, p. 5648.
Lee, C., Yang, W., and Parr, R.G., Phys. Rev., 1988, vol. 37, p. 785.
Bulankin, R.P., Tuzhikov, O.I., and Khardin, A.P., Trudy Volgogr. politekhn. inst. Ser. khim. i khim. tekhnol., 1971, p. 66.
Pudovik, A.N., Konovalova, I.V., Romanov, G.V., Pozhidaev, V.M., Anoshina, N.P., and Lapin, A.A., Zh. Obshch. Khim., 1978, vol. 48, p. 1001.
Reuter, M., and Beermann, C., US Patent no. 3927112, 1975.
Reuter, M., and Rupp, W., US Patent no. 3927113, 1975.
Lippsmeier, B., Hestermann, K., and Reuter, M., US Patent no. 4020110, 1977.
Lippsmeier, B., Hestermann, K., and Reuter, M., US Patent no. 4028421, 1977.
Bright, J.H., US Patent no. 4049719, 1977.
Lin, K., US Patent no. 3732316, 1973.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.I. Grekov, A.O. Litinskii 2014, published in Zhurnal Organicheskoi Khimii, 2014, Vol. 50, No. 8, pp. 1106–1111.
Rights and permissions
About this article
Cite this article
Grekov, L.I., Litinskii, A.O. Quantum-chemical investigation of thermal transformations of tris(hydroxymethyl)phosphine. Russ J Org Chem 50, 1087–1092 (2014). https://doi.org/10.1134/S107042801408003X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107042801408003X