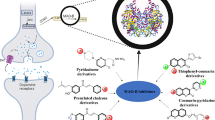
Abstract
Sterically hindered amines such as triacetonamine and certain closely related analogs have been applied in medicine, pharmacology and industry. Various methods of synthesis of 2,2,6,6-tetramethylpiperidin-4-one (triacetonamine) derivatives start generally with acetone, phorone, piperidine N-oxides, piperidine alcohols, and 4-dimethylamine piperidine derivatives. Physical properties of triacetonamine including density, boiling point, flash point, and melting point have been determined. Reactions of triacetonamine derivatives with various organic reagents are also summarized. Triacetonamine derivatives react via three functional groups including carbonyl, methylenes adjacent to the carbonyl group, and NH. Some other miscellaneous reactions are presented. Conformation of triacetonamine is described. Theoretical models for the conformations of triacetonamine have been developed by quantum and molecular mechanics methods. Triacetonamine demonstrates different types of biological activities, such as antialzheimer, antifungal, antimicrobial, anti-HIV, anticancer, antioxidant, P38 kinase inhibitor, DNA labelling, antispasmodic, and psychotropic, and high ganglionic blocking.

















Similar content being viewed by others
Change history
20 June 2020
Erratum
REFERENCES
Smirk, F.H., and Hodge, J.V.J., Chin. Pharm. Therap., 1960, vol. 1, p. 610.
Keana, T.F.W., Chem. Rev., 1978, vol. 78, p. 37. https://doi.org/10.1021/cr60311a004
Guziec, Jr.F.S. and Torres, F.F., J. Org. Chem., 1993, vol. 58, p. 1604. https://doi.org/10.1021/jo00058a055
Hook, R., Ind. Eng. Chem. Res., 1997, vol. 36, p. 1779. https://doi.org/10.1021/ie9605589
Francis, F., J. Chem. Soc., 1927, p. 2897. https://doi.org/10.1039/JR9270002896
Haruna, T., Nishimura, A., and Sugibuchi K., Eur. Patent 0152935, 1985; Chem. Abstr., 1986, vol. 104, no. 88440.
Sosnovsky, G., Lukszo, J., Brasch, R.C., Eriksson, U.G., and Tozer, T.N., J. Med. Chem., 1989, vol. 24, p. 241. https://doi.org/10.1016/0223-5234(89)90005-6
Frolow, O.B., Bode, E., and Engels, J.W., Nucleosides, Nucleotides and Nucleic Acids, 2007, vol. 26, p. 655. https://doi.org/10.1080/15257770701490522
Nakamura, K., Ishiyama, K., Ikai, H., Kanno, T., Sasaki, K., Niwano, Y., and Kohno, M., J. Clin. Biochem. Natur., 2011, vol. 49, no. 2, p. 87. https://doi.org/10.3164/jcbn.10-125
Chengguo, S., Bingcheng, H., and Zuliang, L., Heterocycl. Chem., 2012, vol. 23, no. 3, p. 295. https://doi.org/10.1002/hc.21017
Francavilla, C., Turtle, E.D., Kim, B., O’Mahony, D.J.R., Shiau, T.P., Low, E., Alvarez, N.J., Celeri, C.E., D’Lima, L., Friedman, L.C., Ruado, F.S., Xu, P., Zuck, M.E., Anderson, M.B., Najafi, R., and Jain, R.K., Bioorg. Med. Chem. Let., 2011, vol. 21, p. 3029. https://doi.org/10.1016/j.bmcl.2011.03.035
Dickerman, S.C. and Lindwall, H G., J. Org. Chem., 1949, vol. 14, no. 4, p. 530. https://doi.org/10.1021/jo01156a005
Robertson, J.E., Biel, J.H., and Dierro, F., J. Chem. Soc., 1963, vol. 6, p. 381.
Freter, K., J. Org. Chem., 1975, vol. 40, p. 2525. https://doi.org/10.1021/jo00905a023
Galera, E., szykula, J., and Zabza, A., Liebigs Annalen der Chemie, 1987, vol. 9, p. 777. https://doi.org/10.1002/jlac.198719870827
Xiaopeng, F., Shuai, L., Xilong, Y., Xiaobao, D., and Ligong, C., Catal. Comm., 2010, vol. 11, p. 960. https://doi.org/10.1016/j.catcom.2010.04.012
Frantisek, V., Papalova, A., Manasek, Z., Luston, J., and Hercegova, G., Chem. Abstr., 1988, vol. 109, no. 170231t.
Frantisek, V., Bielikova, A., Vassova, G., Luston, J., Romanov, A., Manasek, Z., and Borsig, E.J., Chem. Abstr., 1988, vol. 109, no. 232108p.
Frantisek, V., Manasek, Z., Papalova, A., and Hercegova, G., Chem. Abstr., 1988, vol. 109, no. 190228v.
Vignali, G., Scrima, R., and Borzatta, V., Eur. Patent 508940; Chem. Abstr., 1992, vol. 117, nos. 49972s, 27964p.
Wanicek, H., Gruber, H., and Greber, G., Die Angew. Makromol. Chem., 1993, vol. 213, p. 207. https://doi.org/10.1002/apmc.1993.052130118
Kagan, E.Sh. and Zhukova, I.Yu., Russ. J. Electrochem., 2000, vol. 36, no. 2, p. 203.
Rohbogner, C J., Wunderlich, S.H., Clososki, G C., and Knochel, P., Eur. J. Org. Chem., 2009, vol. 11, p. 1781. https://doi.org/10.1002/ejoc.200801277
Matsuki, Y., Maly, T., Quari, O., Karoui, H., Moigne, F., Rizzato, E., Lyubenova, S., Herzfeld, J., Prisner, J., Tordo, P., and Griffin, G.R., Angew. Chem., Inter. Ed., 2009, vol. 48, p. 4996. https://doi.org/10.1002/anie.200805940
Dobbs, A.P., Jones, P., Penny, M.J., and Rigby, S.E., Tetrahedron, 2009, vol. 65, p. 5271. https://doi.org/10.1016/j.tet.2009.04.078
Nesvadba, P., Bugnon, L., Marie, P., and Novak, P., Chem. Mater, 2010, vol. 22, p. 783. https://doi.org/10.1021/cm901374u
Meng, S., Xi, Du., Huabang, W., Zhiwei, W., Yang, L., and Ligong, C., Catal. Lett., 2011, vol. 141, p. 1703.
Narumi, T., Arai, H., Yoshimura, K., Harada, S., Nomura, W., Matsushita, S., and Tamamura, H., Bioorg. Med. Chem., 2011, vol. 19, p. 6735. https://doi.org/10.1016/j.bmc.2011.09.045
Wong, P.Y., Chow, W.K., Chung, K.H., Lau, C.P., and Kwong, F.Y., Chem. Commun., 2011, vol. 47, p. 8328. https://doi.org/10.1039/c1cc12240a
Ricci, A., Marinello, J., Bortolus, M., Saanchez, A., Grandas, A., Pedroso, E., Pommier, Y., Capranico, G., Maniero, A.L., and Zagotto, G., J. Med. Chem., 2011, vol. 54, p. 1003. https://doi.org/10.1021/jm101232t
Zakrzewski, J. and Krawczyk, M., Biorg. Med. Chem. Lett., 2011, vol. 21, p. 514. https://doi.org/10.1016/j.bmcl.2010.10.092
Dane, E., Corzilius, B., Rizzato, E., stocker, P., Maly, T., Smith, A., Griffin, R., Quari, O., Tordo, P., and Swager, T., J. Org. Chem., 2012, vol. 77, p. 1789. https://doi.org/10.1021/jo202349j
Ren, X., Kocer, H., Worley, S., Broughton, R., and Huang, T., J. App. Polym. Sci., 2013, p. 3192. https://doi.org/10.1002/app.37731
Rozantser, E.G., Bakin, I.I., Kagan, E.Sh., Zhdanov, R.I., and Zhvlin, V.M., J. Chem. Res. Synopses, 1979, vol. 8, p. 260.
Mori, H., Ohara, M., and Kwan, T., Chem. Pharm. Bull., 1980, vol. 28, p. 3178. https://doi.org/10.1248/cpb.28.3178
Myshikina, L.A., Stoyanovich, F.M., Gol’dfarb, Ya.L., and Kazarina, E.K., Izv. Akad. Nauk SSSR, Ser. Khim., 1986, vol. 35, no. 12, p. 2511.
Brik, M.E., Syn. Comm., 1990, vol. 20, p. 597. https://doi.org/10.1080/00397919008244909
Zhukova, I.Yu., Kagan, E.Sh., and Smirnov, V.A., Zh. Org. Khim., 1993, vol. 29, p. 751.
Elinson, M.N., Dorofeev, A.S., Feducovich, S.K., Nasybullin, R.F., Litvin, E.F., Kopyshev, M.V., and Nikishin, G.I., Tetrahedron, 2006, vol. 62, p. 8021. https://doi.org/10.1016/j.tet.2006.06.031
Sato, S., Tsunoda, M., Suzuki, M., Kutsuna, M., Takido, K., Shinda, M., Mizuguchi, H., Obara, H., and Ohya, H., Spectrochim. Acata A, 2009, vol. 71, p. 2030. https://doi.org/10.1016/j.saa.2008.07.045
Lutz, W.B., Lazarus, S., and Meltzer, R.I., J. Org. Chem., 1962, p. 1695. https://doi.org/10.1021/jo01052a050
Lind, H. and Winkler, T., Tetrahedron Lett., 1980, vol. 21, p. 119. https://doi.org/10.1016/S0040-4039(00)93639-6
Kaliska, V., Toma, S., and Tkac, A., Chem. Papers, 1988, vol. 42, p. 243.
Murray, R.W. and Singh, M., Tetrahedron Lett., 1988, vol. 29, no. 37, p. 4677. https://doi.org/10.1016/S0040-4039(00)80578-X
Brik, M.E., Tetrahedron Lett., 1995, vol. 36, no. 31, p. 5519. https://doi.org/10.1016/0040-4039(95)01053-K
Zakrzewski, J., Syn. Comm., 1988, vol. 18, p. 2135. https://doi.org/10.1080/00397918808068284
Smythe, M.L., Nakaie, C.R., and Marshall, G.R., J. Am. Chem. Soc., 1995, vol. 117, p. 10555. https://doi.org/10.1021/ja00147a018
Kostyanovsky, R.G., Elnalanov, Y.I., Chervin, I.I., Kanovalikhin, S.V., Zolotoc, A.B., and Atovnyan, L.O., Mendeleev Comm., 1996, vol. 6, no. 4, p. 143. https://doi.org/10.1070/MC1996v006n04ABEH000619
Sakai, K., Yamada, K.I., Yamasaki, T., Kinoshita, Y., Mito, F., and Utsumi, H., Tetrahedron, 2010, vol. 66, p. 2311. https://doi.org/10.1016/j.tet.2010.02.004
Krinitskaya, L.A., Russ. Chem. Bull., 2007, vol. 56, no. 3, p. 527.
Blizzard, T.A., Chen, H.Y., Wu, I.Y., Kim, S., Mortko, C. J., Variankava, N., and Chin, A., PCT Int. Appl. No.2008039420
Sato, S., Tsunoda, M., Suzuki, M., Takidouchi, K., Mizuguchi, H., Obara, H., and Ohya, H., Spectrochimica Acta A, 2009, vol. 71, p. 2030. https://doi.org/10.1016/j.saa.2008.07.045
Navajas, C.Ch., Montero, L.A., and Serna, B.La., J. Comp. Aided Mol. Des., 1990, vol. 4, p. 403.
Klemchuk, P.P., ACS symposium Series, American Chemical Society, Washington, DC, June 14, 1985, vol. 280, p. 1. https://doi.org/10.1021/bk-1985-0280.ch001
Wanicek, H., Gruber, H., and Greber, G., Die Angewandte Makromolekulare Chemie, 1993, vol. 213, no. 1, p. 207. https://doi.org/10.1002/apmc.1993.052130118
Bretherick, L., Lee, G.E., Lunt, E., wragg, W.R., and Edge, N.D., Nature, 1959, vol. 184, p. 1707. https://doi.org/10.1038/1841707a0
Kats, M.M., Lavretskaya, E.F., Chervin, I.I., El’natashov, Yu.I., and Kostyanovskii, R.G., Pharm. Chem. J., 1987, vol. 21, no. 6, p. 411.
Ricci, A., Marinello, J., Sanchez, A., Grandas, A., Pedroso, E., Pommier, Y., Gapranico, Maniero, A.L., and Zagotto, G., J. Med. Chem., 2011, vol. 54, no. 4, p. 1003. https://doi.org/10.1021/jm101232t
Hunt, J.A., Kallashi, F., Ruzek, R.D., Sinclair, P.J., Ita, I., Mccormick, S.X., Pivnichny, J.V., Hop, C.E.C.A., Kumar, S., Wang, Z., O’Keefe, S.J., O’Neill, E.A., Porter, G., Thompson, J.E., Woods, A., Zallere, D.M., and Doherty, J.B., Bioorg. Med. Chem. Lett., 2003, vol. 13, p. 467. https://doi.org/10.1016/S0960-894X(02)00990-3
Weinberg, A., Nylander, K.D., Yan, C., Ma, L., Hsia, C.J.C., Tyarin, V.A., Kagan, V.E., and Schot, N.F., Brain Res., 2004, vol. 1012, p. 13. https://doi.org/10.1016/j.brainres.2004.03.048
Frolow, O., Bode, B.E., and Engels, J.W., Nucleosides, Nucleotides and Nucleic Acids, 2007, vol. 26, p. 655. https://doi.org/10.1080/15257770701490522
Kumamoto, T., Suzuki, K., Kim, S.K., Hoshino, K., Ueno, K., Fukuzumi, M., and Ishikawa, T., Helvetica Chemica Acta, 2010, vol. 93, p. 2109. https://doi.org/10.1002/hlca.201000076
Soliman, H.A., Yousif, M.N.M., Said, M.M., Hassan, N.A., Ali, M.M., Awad, H.M., and Abdel-Megeid, F.M.E., Der Pharma Chemica, 2014, vol. 6, no. 3, p. 394.
Fayed, A.A., Bahashwan, S.A., Yousif, M.N.M., Yousif, N.M., and Gad, F.A., Russ. J. Gen. Chem., 2019, vol. 89, no. 9, p. 1887. https://doi.org/10.1134/S1070363219090251
Yousif, M.N.M., Nassar, I.F., Yousif, N.M., Awad, H.M., and El-Sayed, W.A., Russ. J. Gen. Chem., 2019, vol. 89, no. 8, p. 1673. https://doi.org/10.1134/S1070363219080218
Fayed, A.A., Yousif, M.N.M., Abdelgawad, T.T., Amr, A.E., Yousif, N.M., and Gad, F.A., Chem. Heterocycl. Compd., 2019, vol. 55, no. 8, p.773. https://doi.org/10.1007/s10593-019-02534-1
Nemr, M.T.M., Yousif, M.N.M., and Barciszewski, J., Archiv Der Pharmazie, 2019, vol. 352, no. 80, p. 1. https://doi.org/10.1002/ardp.201900062
Yousif, M.N.M., Fayed, A.A., and Yousif, N.M., Egypt. J. Chem., 2019, vol. 62, no. 8, p. 1759. https://doi.org/10.21608/ejchem.2019.7103.1586
Fayed, A.A. and Yousif, M.N.M., Russ. J. Gen. Chem., 2019, vol. 89, no. 6, p. 1209. https://doi.org/10.1134/S1070363219060173
Yousif, M.N.M., Hussein, H.A.R., Yousif, N.M., ElManawaty, M.A., and El-Sayed, W.A., J. Appl. Pharm. Sci., 2019, vol. 9, no. 1, p. 6. https://doi.org/10.7324/JAPS.2019.90102
Yousif, M.N.M., Fayed, A.A., and Yousif, N.M., Der Pharma Chemica, 2018, vol. 10, no. 8, p. 105.
Yousif, M.N.M., El-Sayed, W.A., Abbas, H.S., Awad, H.M., Yousif, N.M., J. Appl. Pharm. Sci., 2017, vol. 7, no. 11, p. 21. https://doi.org/10.7324/JAPS.2017.71104
El-Gazzar, A.B.A., El-Enany, M.M., and Mahmoud, M.N., Bioorg. Med. Chem., 2008, vol. 16, p. 3261. https://doi.org/10.1016/j.bmc.2007.12.012
Narumi, T., Arai, H., Yoshimura, K., Harada, S., Nomura, W., Matsushita, S., Tamamura, H., Bioorg. Med. Chem., 2011, vol. 19, p. 6735. https://doi.org/10.1016/j.bmc.2011.09.045
Francavilla, C., Turtle, E.D., Kim, B., O’Mahony, D.J.R., Shian, T.P., Low, E., Alvarez, N.J., Celeri, C.E., D’Lima, L., Friedman, L.C., Ruado, F.S., Xu, P., Zuck, M.E., Anderson, M.B., Najafi, R.R., and Jain, R.K., Bioorg. Med. Chem. Lett., 2011, vol. 21, p. 3029. https://doi.org/10.1016/j.bmcl.2011.03.035
Zakrzewski, J. and Krawczyk, M., Bioorg. Med. Chem. Lett., 2011, vol. 21, p. 514. https://doi.org/10.1016/j.bmcl.2010.10.092
Kalai, T., Altman, R., Maezawa, I., Balog, M., Morisseau, C., Petriova, J., Hammock, B.D., Jin, L.W., Trudell, T.R., Voss, J.C., and Hideg, K., Eur. J. Med. Chem., 2014, vol. 77, p. 343. https://doi.org/10.1016/j.ejmech.2014.03.026
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Supplementary material
Rights and permissions
About this article
Cite this article
Yousif, M.N.M., Soliman, H.A., Said, M.M. et al. Synthesis and Biological Activity of Triacetonamine. Russ J Gen Chem 90, 460–469 (2020). https://doi.org/10.1134/S1070363220030202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220030202