Abstract
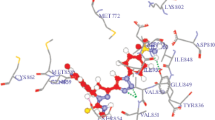
Novel 5-{(1-[(1-phenyl-1H-1,2,3-triazol-4-yl)methyl]-1H-indol-3-yl)methylene}pyrimidine-2,4,6-(1H,3H,5H)trione derivatives (5a–5k) were synthesized by the click reaction. All compounds 5a–5k were characterized by 1H and 13C NMR, IR and Mass spectra and evaluated for their in vitro anticancer activity against cervical cancer cell lines. Among all, compound 5e (IC50 = 6.76 μM), shown high inhibitory activity. Docking analysis of all the compounds with the Lipid kinase PI3K-α revealed that the compound 5e fitted well in the active site pocket, showing the best docking score (LibDock) of 123.274.
Similar content being viewed by others
References
De Simone, R.W., Currie, K.S., Mitchell, S.A., Darrow, J.W., and Pippin, D.A., Comb. Chem. High. Throughput Screen., 2004, vol. 7, p. 473. doi 10.2174/1386207043328544
Wilson and Gisvold’s Textbook of Organic Medicinal Pharmaceutical Chemistry, Delgado, J.N., Remers, W.A., and Lippincott, J.B., Eds., Philadelphia, Pa, USA: Williams & Wilkins, 9 ed., 1991.
Singh, P., Kaur, M., and Verma, P., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 3054. doi 10.1016/j.bmcl.2009.04.014
Sokmen, B.B., Ugras, S., Sarikaya, H.Y., Ugras, H.I., Yanardag, R., Appl. Biochem. Biotechno. 2013, vol. 171, p. 2030. doi 10.1007/s12010-013-0486-6
Khan, K.M., Ali, M., Ajaz, A., Perveen, S., and Choudhary, M.I., Lett. Drug. Des. Discov., 2008, vol. 5, p. 286. doi 10.2174/157018008784619889
Ashnagar, A., Naseri, N.G., and Sheeri, B., Chin. J. Chem., 2007, vol. 25, p. 382. doi 10.1002/cjoc.200790073
Agarwal, A., Lata, S., Saxena, K., Srivastava, V., and Kumar, A., Eur. J. Med. Chem, 2006, vol. 41, p. 1223. doi 10.1016/j.ejmech.2006.03.029
Jursic, B.S., Douelle, F., and Stevens, E.D., Tetrahedron., 2003, vol. 59, p. 3427. doi 10.1016/S0040-4020(03)00489-7
Reddy, Y.T., Sekhar, K.R., Sasi, N., Reddy, P.N., Freeman, M.L., and Crooks, P.A., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 600. doi 10.1016/j.bmcl.2009.11.082
Wang, J., Medina, C., Radomski, M.W., and Gilmer, J.F., Bioorg. Med. Chem., 2011, vol. 19, p. 4985. doi 10.1016/j.bmc.2011.06.055
Andreani, A., Burnelli, S., Granaiola, M., Leoni, A., Locatelli, A., Morigi, R., Rambaldi, M., Varoli, L., Landi, L., Prata, C., Berridge, M.V., Grasso, C., Fiebig, H.H., Kelter, G., Burger, A.M., and Kunkel, M.W., J. Med. Chem., 2008, vol. 51, p. 4563. doi 10.1021/jm800194k
Al Osaimi, A.G., Ali, R.S., and Saad, H.A., Russ. J. Gen. Chem., 2017, vol. 87, p.1246. doi 10.1134/S1070363217060202
Mascal, M., Modes, K.V., and Durmus, A., Angew. Chem. Int. Ed. Engl., 2011, vol. 50, p. 4445. doi 10.1002/anie.201006423
Velezheva, V.S., Brennan, P.J., Marshakov, V.Y., Gusev, D.V., Lisichkina, I.N., Peregudov, A.S., Tchernousova, L.N., Smirnova, T.G., Andreevskaya, S.N., and Medvedev, A.E., J. Med. Chem. 2004, vol. 47, p. 3455. doi 10.1021/jm030479g
Narayana, B., Ashalatha, B.V., Vijayaraj, K.K., Fernandes, J., and Sarojini, B.K., Bioorg. Med. Chem., 2005, vol. 13, p. 4638. doi 10.1016/j.bmc.2005.04.068
Regina, G.L., Coluccia, A., Piscitelli, F., Bergamini, A., Sinistro, A., Cavazza, A., Maga, G., Samuele, A., Zanoli, S., Novellino, E., Artico, M., and Silvestri, R., J. Med. Chem., 2007, vol. 50, p. 5034. doi 10.1021/jm070488f
Tunca, G.A., Nilufer, Y., Tulay, C., Sureyya. O., Lett. Drug. Des. Discov., 2017, vol. 14, p. 380. doi 10.2174/1570180813666161020165623
Sechi, M., Derudas, M., Dallocchio, R., Dessi, A., Bacchi, A., Sannia, L., Carta, F., Palomba, M., Ragab, O., Chan, C., Shoemaker, R., Sei, S., Dayam, R., and Neamati, N., J. Med. Chem., 2004, vol. 47, p. 5298. doi 10.1021/jm049944f
Sowjanya, T., Jayaprakash Rao, Y., and Murthy, N.Y.S., Russ. J. Gen. Chem., 2017, vol. 87, p. 1864. doi 10.1134/S1070363217080357
Yadav, P., Lal, K., Kumar, A., Guru, S.K., Jaglan, S., and Bhushan, S., Eur. J. Med. Chem., 2017, vol. 126, p. 944. doi 10.1016/j.ejmech.2016.11.030
Kant, R., Singh, V., Nath, G., Awasthi, S.K., and Agarwal, A., Eur. J. Med. Chem., 2016, vol. 124, p. 218. doi 10.1016/j.ejmech.2016.08.031
Sathish Kumar, B., Veena, B.S., Anantha Lakshmi, P.V., Kamala., L., and E. Sujatha., Russ. J. Bioorg. Chem., 2017, vol. 43, p. 589. doi 10.1134/S1068162017050120
El-Sayed, W.A., Khalaf, H.S., and Mohamed, S.F., Russ. J. Gen. Chem., 2017, vol. 87, p. 2444. doi 10.1134/S1070363217100279
Shafi, S., Alam, M.M., Mulakayala, N., Mulakayala, C., Vanaja, G., Kalle, A.M., Pallu, R., and Alam, M.S., Eur. J. Med. Chem. 2012, vol. 49, p. 324. doi 10.1016/j.ejmech.2012.01.032
Shanmugavelan, P., Nagarajan, S., Kumar M.S., Ponnuswamy, A., Yogeeswari, P., and Sriram, D., Bioorg. Med. Chem. Lett. 2011, vol. 21, p. 7273. doi 10.1016/j.bmcl.2011.10.048
Da Silva Fde, C., De Souza, M.C., Frugulhetti., Castro, H.C., Souza, S.L., De Souza, T.M., Rodrigues, D.Q., Souza, A.M., Abreu, P.A., Passamani, F., Rodrigues, C.R., and Ferreira, V.F., Eur. J. Med. Chem., 2009, vol. 44, p. 373. doi 10.1016/j.ejmech.2008.02.047
Kelley, J.L., Koble, C.S., Davis, R.G., McLean, E.W., Soroko, F.E., and Cooper, B.R., J. Med. Chem., 1995, vol. 38, p. 4131. doi 10.1021/jm00020a030
Aher, N.G., Pore, V.S., Mishra, N.N., Kumar, A., Shukla, P.K., Sharma, A., and Bhat, M.K., Bioorg. Med. Chem. Lett., 2009, vol. 19, p. 759. doi 10.1016/j.bmcl.2008.12.026
Wang, X., Dai, Z.C., Chen, Y.F., Cao, L.L., Yan, W., Li, S.K., Wang, J.X., Zhang, Z.G., and Ye, Y.H., Eur. J. Med. Chem., 2017, vol. 126, p. 171. doi 10.1016/j.ejmech.2016.10.006
Sathish Kumar, B., and Anantha Lakshmi, P.V., Russ. J. Gen. Chem., 2017, vol. 87, p. 1057. doi 10.1134/S1070363217050279
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Kumar, A., Sathish Kumar, B., Sreenivas, E. et al. Synthesis, Biological Evaluation, and Molecular Docking Studies of Novel 1,2,3-Triazole Tagged 5-[(1H-Indol-3-yl)methylene]pyrimidine-2,4,6(1H,3H,5H)trione Derivatives. Russ J Gen Chem 88, 587–595 (2018). https://doi.org/10.1134/S1070363218030313
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363218030313