Abstract
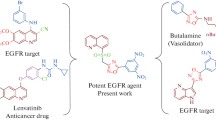
ctive: Benzothiazole and imidazo[2,1-b][1,3,4]thiadiazole derivatives are the most important pharmacophores and intermediates for making drugs. This study focuses on the preparation and anti-cancer activity of novel benzothiazole-based imidazo[2,1-b][1,3,4]thiadiazole scaffolds. Methods: The synthesized benzothiazolebased imidazo[2,1-b][1,3,4]thiadiazole scaffolds (Va–Vi) were evaluated. The anticancer activity of (Va–Vi) against MCF-7 and A549 cell lines was determined using the MTT assay and screened with in silico molecular docking studies. Results and Discussion: Compounds 2-(6-(4-chlorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl)benzo[d]thiazole (Vb), 2-(6-(4-bromophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl)benzo[d]thiazole (Vd), and 2-(6-(4nitrophenyl)imidazo[2,1-b][1,3,4]thiadiazol-2-yl)benzo[d]thiazole (Vh) exhibited the most potent anticancer activity against MCF-7 and A549 cancer cell lines. Molecular docking studies of all synthesized compounds and erlotinib were also carried out on the EGFR receptor, showing that compounds (Vb), (Vd), and (Vh) had significantly higher binding scores and inhibitory constants than the reference drug erlotinib. Conclusions: It has been observed that the substitution on the 4-chlorophenyl ring (Vb), 4-bromophenyl ring (Vd), and 4-nitrophenyl ring (Vh) is important for maintaining their anticancer activity. The outcomes of the kinase inhibitory assay of these significant (Vb), (Vd), and (Vh) hybrids against the tyrosine kinase EGFR strongly corroborated the in vitro anticancer findings and the in silico docking investigations. The investigation demonstrated that the newly synthesized compounds have the potential as anticancer agents and provided leads for further development.






Similar content being viewed by others
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Ewes, W.A., Elmorsy, M.A., El-Messery, S.M., and Nasr, M.N.A., Bioorg. Med. Chem., 2020, vol. 28, p. 115373. https://doi.org/10.1016/j.bmc.2020.115373
Hassan, G.S., Georgey, H.H., Mohammed, E.Z., George, R.F., Mahmoud, W.R., and Omar, F.A., Eur. J. Med. Chem., 2021, vol. 218, p. 113389. https://doi.org/10.1016/j.ejmech.2021.113389
Shafei, A., El-Bakly, W., Sobhy, A., Wagdy, O., Reda, A., Aboelenin, O., Marzouk, A., El Habak, K., Mostafa, R., Ali, M.A., and Ellithy, M., Biomed. Pharmacother., 2017, vol. 95, pp. 1209−1218. https://doi.org/10.1016/j.biopha.2017.09.059
Hashem, H.E., Amr, A.E.G.E., Nossier, E.S., Anwar, M.M., and Azmy, E.M., ACS Omega, 2022, vol. 7, pp. 7155–7171. https://doi.org/10.1021/acsomega.1c06836
Kim, M., Baek, M., and Kim, D.J., Curr. Pharm. Des., 2017, vol. 23, pp. 4226−4246. https://doi.org/10.2174/1381612823666170616082125
Rezki, N., Almehmadi, M.A., Ihmaid, S., Shehata, A.M., Omar, A.M., Ahmed, H.E., and Aouad, M.R., Bioorg. Chem., 2020, vol. 103, p. 104133. https://doi.org/10.1016/j.bioorg.2020.104133
Othman, I.M.M., Alamshany, Z.M., Tashkandi, N.Y., GadElkareem, M.A.M., Anwar, M.M., and Nossier, E., Bioorg. Chem., 2021, vol. 114, p. 105078. https://doi.org/10.1016/j.bioorg.2021.105078
Abd El-Meguid, E.A., Moustafa, G.O., Awad, H.M., Zaki, E.R., and Nossier, E.S., J. Mol. Struct., 2021, vol. 1240, p. 130595. https://doi.org/10.1016/j.molstruc.2021.130595
Ahmed, M.F., Santali, E.Y., El-Deen, E.M.M., Naguib, I.A., and El-Haggar, R., Bioorg. Chem., 2021, vol. 106, p. 104473. https://doi.org/10.1016/j.bioorg.2020.104473
Eshacharyulu, P., Ponnusamy, M.P., Haridas, D., Jain, M., Ganti, A.K., and Batra, S.K., Exp. Opin. Ther. Target., 2012, vol. 16, pp. 15−31. https://doi.org/10.1517/14728222.2011.648617
Deng, X., Tan, X., An, T., Ma, Q., Jin, Z., Wang, C., Meng, Q., and Hu, C., Molecules, 2019, vol. 24, p. 682. https://doi.org/10.3390/molecules24040682
Meier, F., Schittek, B., Busch, S., Garbe, C., Smalley, K., Satyamoorthy, K., Li, G., and Herlyn, M., Front. Biosci.– Landmark, 2005, vol. 10, pp. 2986–3001.
Asif, M. and Imran, M., Mini-Rev. Org. Chem., 2021, vol. 18, pp. 1086–1097. https://doi.org/10.2174/1570193X17999201127110214
Kurt, A.H., Ayaz, L., Ayaz, F., Seferoglu, Z., and Nural, Y., Curr. Org. Syn., 2022, vol. 19, pp. 772–796. https://doi.org/10.2174/1570179419666220330001036
Haider, K., Rehman, S., Pathak, A., Najmi, A.K., and Yar, M.S., Arch. der Pharmazie, 2021, vol. 354, p. 2100246. https://doi.org/10.1002/ardp.202100246
Djuidje, E.N., Barbari, R., Baldisserotto, A., Durini, E., Sciabica, S., Balzarini, J., Liekens, S., Vertuani, S., and Manfredini, S., Antioxidants, 2022, vol. 11, p. 407. https://doi.org/10.3390/antiox11020407
Kumar, G. and Singh, N.P., Bioorg. Chem., 2021, vol. 107, p. 104608. https://doi.org/10.1016/j.bioorg.2020.104608
Venugopala, K.N., Chandrashekharappa, S., Pillay, M., Bhandary, S., Kandeel, M., Mahomoodally, F.M., Morsy, M.A., Chopra, D., Aldhubiab, B.E., Attimarad, M., and Alwassil, O.I., Med. Chem., 2019, vol. 15, pp. 311–326. https://doi.org/10.2174/1573406414666180703121815
Thakkar, S.S., Thakor, P., Ray, A., Doshi, H., and Thakkar, V.R., Bioorg. Med. Chem., 2017, vol. 25, pp. 5396–5406. https://doi.org/10.1016/j.bmc.2017.07.057
Asiri, Y.I., Alsayari, A., Muhsinah, A.B., Mabkhot, Y.N., and Hassan, M.Z., J. Pharm. Pharmacol., 2020, vol. 72, pp. 1459–1480. https://doi.org/10.1111/jphp.13331
Bhagdev, K. and Sarkar, S., Annal. Rom. Soc. Cell Biol., 2021, vol. 25, pp. 20269–20285. http://www.annalsofrscb.ro/index.php/journal/article/view/9199
Nath, R., Yar, M.S., Pathania, S., Grover, G., Debnath, B., and Akhtar, M.J., J. Mol. Struct., 2021, vol. 1228, p. 129742. https://doi.org/10.1016/j.molstruc.2020.129742
Singh, M.K., Tilak, R., Nath, G., Awasthi, S.K., and Agarwal, A., Eur. J. Med. Chem., 2013, vol. 63, pp. 635–644. https://doi.org/10.1016/j.ejmech.2013.02.027
Irfan, A., Batool, F., Zahra Naqvi, S.A., Islam, A., Osman, S.M., Nocentini, A., Alissa, S.A., and Supuran, C.T., J. Enzyme Inhib. Med. Chem., 2020, vol. 35, pp. 265–279. https://doi.org/10.1080/14756366.2019.1698036
Saeed, S., Rashid, N., Jones, P.G., Ali, M., and Hussain, R., Eur. J. Med. Chem., 2010, vol. 45, pp. 1323–1331. https://doi.org/10.1016/j.ejmech.2009.12.016
Abdelgawad, M.A., Belal, A., Omar, H.A., Hegazy, L., and Rateb, M.E., Arch. der. Pharmazie, 2013, vol. 346, pp. 534–541. https://doi.org/10.1002/ardp.201300044
Gabr, M.T., El-Gohary, N.S., El-Bendary, E.R., and El-Kerdawy, M.M., Med. Chem. Res., 2015, vol. 24, pp. 860–878. https://doi.org/10.1007/s00044-014-1114-x
Abdellatif, K.R., Belal, A., El-Saadi, M.T., Amin, N.H., Said, E.G., and Hemeda, L.R., Bioorg. Chem., 2020, vol. 101, p. 103976. https://doi.org/10.1016/j.bioorg.2020.103976
Gabr, M.T., El-Gohary, N.S., El-Bendary, E.R., and El-Kerdawy, M.M., EXCLI J., 2014, vol. 13, pp. 573–585. https://www.excli.de/index.php/excli/article/view/723
Bhongade, B.A., Talath, S., Gadad, R.A., and Gadad, A.K., J. Saudi Chem. Soc., 2016, vol. 20, pp. S463–S475. https://doi.org/10.1016/j.jscs.2013.01.010
Khan, I., Ibrar, A., and Abbas, N., Eur. J. Med. Chem., 2013, vol. 63, pp. 854–868. https://doi.org/10.1016/j.ejmech.2013.01.060
Cristina, A., Leonte, D., Vlase, L., Bencze, L.C., Imre, S., Marc, G., Apan, B., Mogoșan, C., and Zaharia, V., Molecules, 2018, vol. 23, p. 2425. https://doi.org/10.3390/molecules23102425
Dagli, M., Er, M., Karakurt, T., Onaran, A., Alici, H., and Tahtaci, H., ChemistrySelect, 2020, vol. 5, pp. 11753–11763. https://doi.org/10.1002/slct.202002821
Yan Guo, F., Ji Zheng, C., Wang, M., Ai, J., Ying Han, L., Yang, L., Fang Lu, Y., Xuan Yang, Y., Guan Piao, M., Piao, H.R., and Jin, C.M., ChemMedChem, 2021, vol. 16, pp. 2354–2365. https://doi.org/10.1002/cmdc.202100122
Er, M., Özer, A., Direkel, Ş., Karakurt, T., and Tahtaci, H., J. Mol. Struct., 2019, vol. 1194, pp. 284–296. https://doi.org/10.1016/j.molstruc.2019.05.104
Er, M., Ergüven, B., Tahtaci, H., Onaran, A., Karakurt, T., and Ece, A., Med. Chem. Res., 2017, vol. 26, pp. 615–630. https://doi.org/10.1007/s00044-017-1782-4
Taflan, E., Bayrak, H., Er, M., Karaoğlu, Ş.A., and Bozdeveci, A., Bioorg. Chem., 2019, vol. 89, p. 102998. https://doi.org/10.1016/j.bioorg.2019.102998
Dincel, E.D., Gürsoy, E., Yilmaz-Ozden, T., and UlusoyGüzeldemirci, N., Bioorg. Chem., 2020, vol. 103, p. 104220. https://doi.org/10.1016/j.bioorg.2020.104220
Haider, S., Alam, M.S., and Hamid, H., Eur. J. Med. Chem., 2015, vol. 92, pp. 156–177. https://doi.org/10.1016/j.ejmech.2014.12.035
Cascioferro, S, Parrino, B, Petri, G.L., Cusimano, M.G., Schillaci, D., Di Sarno, V., Musella, S., Giovannetti, E., Cirrincione, G., and Diana, P., Eur. J. Med. Chem., 2019, vol. 167, pp. 200–210. https://doi.org/10.1016/j.ejmech.2019.02.007
Turner, S., Myers, M., Gadie, B., Hale, S.A., Horsley, A., Nelson, A.J., Pape, R., Saville, J.F., Doxey, J.C., and Berridge, T.L., J. Med. Chem., 1988, vol. 31, pp. 906–913. https://doi.org/10.1021/jm00400a004
Andreani, A., Leoni, A., Locateur, A., Morigi, R., Rambaldi, M., Simon, W.A., and Senn-Bilfinger, J., Arzneimittelforschung, 2000, vol. 50, pp. 550–553. https://doi.org/10.1055/s-0031-1300247
Palabindela, R., Guda, R., Ramesh, G., Bodapati, R., Nukala, S.K., Myadaraveni, P., Ravi, G., and Kasula, M., J. Mol. Struct., 2023, vol. 1275, p. 134633. https://doi.org/10.1016/j.molstruc.2022.134633
Ramya Sucharitha, E., Kumar Nukala, S., Swamy Thirukovela, N., Palabindela, R., Sreerama, R., and Narsimha, S., ChemistrySelect, 2023, vol. 8, p. e202204256. https://doi.org/10.1002/slct.202204256
Ismael, A.S., Amin, N.H., Elsaadi, M.T., and AbdelRahman, H.M., Bioorg. Chem., 2022, vol. 128, p. 106042. https://doi.org/10.1016/j.bioorg.2022.106042
Park, J.H., Liu, Y., Lemmon, M.A., and Radhakrishnan, R., Biochem. J., 2022, vol. 448, pp. 417–423. https://doi.org/10.1042/BJ20121513
Palabindela, R., Guda, R., Ramesh, G., Myadaraveni, P., Banothu, D., Ravi, G., Korra, R., Mekala, H., and Kasula, M., J. Heterocyc. Chem., vol. 59, pp. 1533–1550. https://doi.org/10.1002/jhet.4488
ACKNOWLEDGMENTS
The authors are thankful to the head of the Department of Pharmaceutical Science at Kakatiya University, Warangal, for providing biological activity data. Grateful to the Director, IICT-Hyderabad, for providing spectral data.
Funding
This work was supported by regular institutional funding, and no additional grants were obtained.
Author information
Authors and Affiliations
Contributions
The author BMR is involved in the methodology, investigation, and data curation. The author MS is involved in the designed chemistry part and manuscript language editing process; the author MH is involved in the molecular docking studies; the author KB is involved in the experimental data; the authors GVRSM and SRB is participated in the in vitro anticancer studies. The author TK is involved in the manuscript language editing process. The author MK is involved in the supervision of manuscript writing etc.
All authors participated in the discussions.
Corresponding author
Ethics declarations
This article does not contain any studies involving patients or animals as test objects.
Informed consent was not required for this article. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Reddy, B.M., Sridhar, M., Himabindu, M. et al. Synthesis and Biological Evaluation of 2-(6-Phenylimidazo[2,1-b][1,3,4]thiadiazol-2-yl)benzo[d]thiazole Derivatives as EGFR Targeting Anticancer Agents. Russ J Bioorg Chem 50, 571–581 (2024). https://doi.org/10.1134/S1068162024020146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162024020146