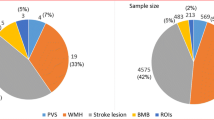
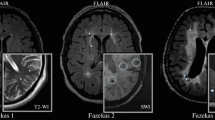
Abstract
Cerebral small vessel disease (cSVD) is the leading cause of vascular cognitive impairments and dementia, cerebral hemorrhages and lacunar strokes, as well as the most common form of asymptomatic vascular brain lesion. Major forms of cSVD are age- and arterial hypertension (AH)-associated arteriolosclerosis and cerebral amyloid angiopathy. The etiologies and the underlying mechanisms of disease development and progression remain unclear for a substantial group of cSVD types. Significant difficulties in the study of this pathology are explained by technical limitations in assessing smallest vessels in vivo. A modified correlation between MRI equivalents and their morphological manifestations in cSVD to use them subsequently as a surrogate marker of lesions in small vessels has allowed clinicians to establish disease progression regularities and the association of the latter with clinical symptoms. This review presents the results of studies showing the clinical significance and role of the leading MRI features in the assessment of disease progression, including white matter hyperintensity (WMH, formerly known as leukoaraiosis), lacunes, enlarged perivascular spaces, and microbleeds. The recognition of MRI features as diagnostic criteria for cSVD was specified by international experts in the Standards for Reporting Vascular Changes on Neuroimaging (the STRIVE criteria). Despite the enormous importance of this standardization for the improvement of concepts about the significance of different factors in the development and understanding of heterogeneity of cSVD forms, this categorization cannot provide for the prediction of the disease course in a particular patient and assess the treatment efficacy in short- and medium-term prospects. One of the approaches to solution was based on the use of diffusion methodologies for assessing a microstructural lesion in the visually unaltered brain matter. The obtained consistent association of the expressiveness of microstructural alterations with clinical impairments substantiates the expediency of multimodal MRI studies aimed to evaluate the pathophysiological mechanisms of disease progression, beginning from the subclinical brain lesion stage.
Similar content being viewed by others
REFERENCES
Pantoni, L., Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges, Lancet Neurol., 2010, vol. 9, no. 7, p. 689. https://doi.org/10.1016/S1474-4422(10)70104-6
Pasi, M., van Uden, I.W., Tuladhar, A.M., et al., White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences, Stroke, 2016, vol. 47, no. 6, p. 1679. https://doi.org/10.1161/STROKEAHA.115.012065
Wardlaw, J.M., Smith, C., and Dichgans, M., Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging, Lancet Neurol., 2013, vol. 12, no. 5, p. 483. https://doi.org/10.1016/S1474-4422(13)70060-7
Gorelick, P.B., Scuteri, A., Black, S.E., et al., Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association, Stroke, 2011, vol. 42, p. 2672. https://doi.org/10.1161/STR.0b013e3182299496
Charidimou, A., Pantoni, L., and Love, S., The concept of sporadic cerebral small vessel disease: a road map on key definitions and current concepts, Int. J. Stroke, 2016, vol. 11, no. 1, p. 6. https://doi.org/10.1177/1747493015607485
Qureshi, A.I., Mendelow, A.D., and Hanley, D.F., Intracerebral haemorrhage, Lancet, 2009, vol. 373, no. 9675, p. 1632. https://doi.org/10.1016/S0140-6736(09)60371-8
Sudlow, C.L. and Warlow, C.P., Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration, Stroke, 1997, vol. 28, p. 491.
Biessels, G.J., Diagnosis and treatment of vascular damage in dementia, Biochim. Biophys. Acta, 2016, vol. 1862, no. 5, p. 869. https://doi.org/10.1016/j.bbadis.2015.11.009
Smallwood, A., Oulhaj, A., Joachim, C., et al., Cerebral subcortical small vessel disease and its relation to cognition in elderly subjects: a pathological study in the Oxford Project to Investigate Memory and Ageing (OPTIMA) cohort, Neuropathol. Appl. Neurobiol., 2012, vol. 38, p. 337. https://doi.org/10.1111/j.1365-2990.2011.01221.x
Verhaaren, B.F., Vernooij, M.W., de Boer, R., et al., High blood pressure and cerebral white matter lesion progression in the general population, Hypertension, 2013, vol. 61, p. 1354. https://doi.org/10.1161/HYPERTENSIONAHA.111.00430
Wardlaw, J.M., Smith, E.E., Biessels, G.J., et al., Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration, Lancet Neurol., 2013, vol. 12, no. 8, p. 822. https://doi.org/10.1016/S1474-4422(13)70124-8
Raina, A., Zhao, X. Grove, M.L., et al., Cerebral white matter hyperintensities on MRI and acceleration of epigenetic aging: the atherosclerosis risk in communities’ study, Clin. Epigenet., 2017, vol. 14, no. 9, p. 21. https://doi.org/10.1186/s13148-016-0302-6
Barkhofa, F. and Scheltensb, P., Imaging of white matter lesions, Cerebrovasc. Dis., 2002, vol. 13. suppl. 2, p. 21. https://doi.org/10.1159/000049146
Fazekas, F., Chawluk, J.B., Alavi, A., et al., MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging, Am. J. Roentgenol., 1987, vol. 149, no. 2, p. 351. https://doi.org/10.2214/ajr.149.2.351
de Leeuw, F.E., de Groot, J.C., Achten, E., et al., Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study: The Rotterdam Scan Study, J. Neurol. Neurosurg. Psychiatry, 2001, vol. 70, p. 9.
Scheltens, P., Barkhof, F., Leys, D., et al., A semiquantitative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging, J. Neurol. Sci., 1993, vol. 114, no. 1, p. 7.
Wahlund, L.O., Agartz, I., Almqvist, O., et al., The brain in healthy aged individuals: MR imaging, Radiology, 1990, vol. 174, no. 3, part 1, p. 675. https://doi.org/10.1148/radiology.174.3.2305048
Longstreth, W.T. Jr., Sonnen, J.A., Koepsell, T.D., et al., Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases, Alzheimer Dis. Assoc. Disord., 2009, vol. 23, p. 291. https://doi.org/10.1097/WAD.0b013e318199fc7a
Prins, N.D., van Straaten, E.C., van Dijk, E.J., et al., Measuring progression of cerebral white matter lesions on MRI: visual rating and volumetrics, Neurology, 2004, vol. 62, no. 9, p. 1533.
Schmidt, R., Schmidt, H., Haybaeck, J., et al., Heterogeneity in age-related white matter changes, Acta Neuropathol., 2011, vol. 122, p. 171. https://doi.org/10.1007/s00401-011-0851-x
Dufouil, C., Chalmers, J., Coskun, O., et al., Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection against Recurrent Stroke Study) Magnetic Resonance Imaging substudy, Circulation, 2005, vol. 112, no. 11, p. 1644. https://doi.org/10.1161/CIRCULATIONAHA.104.501163
Gottesman, R.F., Coresh, J., Catellier, D.J., et al., Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study, Stroke, 2010, vol. 41, no. 1, p. 3. https://doi.org/10.1161/STROKEAHA.109.566992
Maillard, P., Crivello, F., Dufouil, C., et al., Longitudinal follow-up of individual white matter hyperintensities in a large cohort of elderly, Neuroradiology, 2009, vol. 51, p. 209. https://doi.org/10.1007/s00234-008-0489-0
Kloppenborg, R.P., Nederkoorn, P.J., Grool, A.M., et al., Cerebral small-vessel disease and progression of brain atrophy: the SMART-MR study, Neurology, 2012, vol. 79, p. 2029. https://doi.org/10.1212/WNL.0b013e3182749f02
Debette, S. and Markus, H.S., The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis, BMJ, 2010, vol. 341, p. c3666. https://doi.org/10.1136/bmj.c3666
Wardlaw, J.M., Valdés Hernández, M.C., and Muñoz-Maniega, S, What are white matter hyperintensities made of? Relevance to vascular cognitive impairment, J Am Heart Assoc., 2015, vol. 4, no. 6, p. 001140. https://doi.org/10.1161/JAHA.114.001140
Raman, M.R., Kantarci, K., Murray, M.E., et al., Imaging markers of cerebrovascular pathologies: pathophysiology, clinical presentation, and risk factors, Alzheimers Dement. (Amsterdam), 2016, vol. 5, p. 5. https://doi.org/10.1016/j.dadm.2016.12.006
LADIS Study Group, 2001—2011: a decade of the LADIS (LeukoaraiosisAndDISability) study: what have we learned about white matter changes and small-vessel disease? Cerebrovasc. Dis., 2011, vol. 32, no. 6, p. 577. https://doi.org/10.1159/000334498
Herrmann, L.L., Le Masurier, M., and Ebmeier, K.P., White matter hyperintensities in late life depression: a systematic review, J. Neurol. Neurosurg. Psychiatry, 2008, vol. 79, p. 619. https://doi.org/10.1136/jnnp.2007.124651
Wright, C.B., Dong, C., Perez, E.J., et al., Subclinical cerebrovascular disease increases the risk of incident stroke and mortality: The Northern Manhattan Study, J. Am. Heart Assoc., 2017, vol. 6, no. 9. https://doi.org/10.1161/JAHA.116.004069
Windham, B.G., Deere, B., Griswold, M.E., et al., Small brain lesions and incident stroke and mortality: a cohort study, Ann. Int. Med., 2015, vol. 163, no. 1, p. 22. https://doi.org/10.7326/M14-2057
Schretlen, D.J., Testa, S.M., Winicki, J.M., et al., Frequency and bases of abnormal performance by healthy adults on neuropsychological testing, J. Int. Neuropsychol. Soc., 2008, vol. 14, no. 3, p. 436. https://doi.org/10.1017/S1355617708080387
Carmelli, D., DeCarli, C., Swan, G.E., et al., Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins, Stroke, 1998, vol. 29, no. 6, p. 1177.
Verhaaren, B.F., de Boer, R., Vernooij, M.W., et al., Replication study of chr17q25 with cerebral white matter lesion volume, Stroke, 2011, vol. 42, no. 11, p. 3297. https://doi.org/10.1161/STROKEAHA.111.623090
Adib-Samii, P., Rost, N., Traylor, M., et al., 17q25 Locus is associated with white matter hyperintensity volume in ischemic stroke, but not with lacunar stroke status, Stroke, 2013, vol. 44, no. 6, p. 1609. https://doi.org/10.1161/STROKEAHA.113.679936
Tabara, Y., Igase, M., Okada, Y., et al., Association of Chr17q25 with cerebral white matter hyperintensities and cognitive impairment: the J-SHIPP study, Eur. J. Neurol., 2013, vol. 20, no. 5, p. 860. https://doi.org/10.1111/j.1468-1331.2012.03879.x
Lin, Q., Huang, W.Q., and Tzeng, C.M., Genetic associations of leukoaraiosis indicate pathophysiological mechanisms in white matter lesions etiology, Rev. Neurosci., 2015, vol. 26, no. 3, p. 343. https://doi.org/10.1515/revneuro-2014-0082
de Leeuw, F.E., de Groot, J.C., Oudkerk, M., et al., Hypertension and cerebral white matter lesions in a prospective cohort study, Brain, 2002, vol. 125, part 4, p. 765.
Dufouil, C., de Kersaint-Gilly, A., Besancon, V., et al., Longitudinal study on blood pressure and white matter hyperintensities: the EVA MRI cohort, Neurology, 2001, vol. 56, no. 7, p. 921.
Dobrynina, LA., Gnedovskaya, E.V. Sergeeva, A.N., et al., Subclinical cerebral manifestations and changes of brain associated with newly diagnosed asymptomatic arterial hypertension, Ann. Klin. Eksp. Nevrol., 2016, vol. 10, no. 3, p. 26.
Dobrynina, L.A., Gnedovskaya, E.V., Sergeeva, A.N., et al., Changes in the MRI brain picture associated with newly diagnosed asymptomatic arterial hypertension, Ann. Klin. Eksp. Nevrol., 2016, vol. 10, no. 3, p. 33.
Schmidt, R., Fazekas, F., Enzinger, C., et al., Risk factors and progression of small vessel disease-related cerebral abnormalities, J. Neural Transm., Suppl., 2002, vol. 62, p. 47.
Schmidt, R., Enzinger, C., Ropele, S., et al., Progression of cerebral white matter lesions: 6-year results of the Austrian Stroke Prevention Study, Lancet, 2003, vol. 361, p. 2046.
van Leijsen, E.M.C., van Uden, I.W.M., Ghafoo-rian, M., et al., The rise and fall of cerebral small vessel disease—the RUN DMC study, Eur. Stroke J., 2016.
Maillard, P., Fletcher, E., Lockhar, S.N., et al., White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain, Stroke, 2014, vol. 45, no. 6, p. 1721. https://doi.org/10.1161/STROKEAHA.113.004084
Ryu, W.S., Woo, S.H., Schellingerhout, D., et al., Grading and interpretation of white matter hyperintensities using statistical maps, Stroke, 2014, vol. 45, p. 3567. https://doi.org/10.1161/STROKEAHA.114.006662
van Leijsen, E.M.C., de Leeuw, F.E., and Tuladhar, A.M., Disease progression and regression in sporadic small vessel disease—insights from neuroimaging, Clin. Sci., 2017, vol. 131, no. 12, p. 1191. https://doi.org/10.1042/CS20160384
Longstreth, W.T., Jr., Dulberg, C., Manolio, T.A., et al., Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study, Stroke, 2002, vol. 33, no. 10, p. 2376.
Gouw, A.A., van der Flier, W.M., Pantoni, L., et al., On the etiology of incident brain lacunes: longitudinal observations from the LADIS study, Stroke, 2008, vol. 39, no. 11, p. 3083. https://doi.org/10.1161/STROKEAHA.108.521807
Duering, M., Csanadi, E., Gesierich, B., et al., Incident lacunes preferentially localize to the edge of white matter hyperintensities: insights into the pathophysiology of cerebral small vessel disease, Brain, 2013, vol. 136, part 9, p. 2717. https://doi.org/10.1093/brain/awt184
Jokinen, H., Gouw, A.A., Madureira, S., et al., Incident lacunes influence cognitive decline: the LADIS study, Neurology, 2011, vol. 76, no. 22, p. 1872. https://doi.org/10.1212/WNL.0b013e31821d752f
Wright, C.B., Festa, J.R., Paik, M.C., et al., White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility, Stroke, 2008, vol. 39, no. 3, p. 800. https://doi.org/10.1161/STROKEAHA.107.484147
van Dijk, E.J., Prins, N.D., Vrooman, H.A., et al., Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study, Stroke, 2008, vol. 39, no. 10, p. 2712. https://doi.org/10.1161/STROKEAHA.107.513176
Schneider, J.A., Aggarwal, N.T., Barnes, L., et al., The neuropathology of older persons with and without dementia from community versus clinic cohorts, J. Alzheimers Dis., 2009, vol. 18, no. 3, p. 691. https://doi.org/10.3233/JAD-2009-1227
Brundel, M., de Bresser, J., van Dillen, J.J., et al., Cerebral microinfarcts: a systematic review of neuropathological studies, J. Cereb. Blood Flow Metab., 2012, vol. 32, no. 3, p. 425. https://doi.org/10.1038/jcbfm.2011.200
van Veluw, S.J., Zwanenburg, J.J., Engelen-Lee, J., et al., In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI, J. Cereb. Blood Flow Metab., 2013, vol. 33, no. 3, p. 322. https://doi.org/10.1038/jcbfm.2012.196
Auriel, E., Edlow, B.L., Reijmer, Y.D., et al., Microinfarct disruption of white matter structure: a longitudinal diffusion tensor analysis, Neurology, 2014, vol. 83, no. 8, p. 182. https://doi.org/10.1212/WNL.0000000000000579
Deramecourt, V., Slade, J.Y., Oakley, A.E., et al., Staging and natural history of cerebrovascular pathology in dementia, Neurology, 2012, vol. 78, no. 14, p. 1043. https://doi.org/10.1212/WNL.0b013e31824e8e7f
Patel, B. and Markus, H.S., Magnetic resonance imaging in cerebral small vessel disease and its use as a surrogate disease marker, Int. J. Stroke, 2011, vol. 6, no. 1, p. 47. https://doi.org/10.1111/j.1747-4949.2010.00552.x
Knudsen, K.A., Rosand, J., Karluk, D., et al., Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria, Neurology, 2001, vol. 56, no. 4, p. 537.
Iadecola, C., The pathobiology of vascular dementia, Neuron, 2013, vol. 80, no. 4, p. 844. https://doi.org/10.1016/j.neuron.2013.10.008
Poels, M.M., Ikram, M.A., van der Lugt, A., et al., Incidence of cerebral microbleeds in the general population: the Rotterdam Scan Study, Stroke, 2011, vol. 42, no. 3, p. 656. https://doi.org/10.1161/STROKEAHA.110.607184
Lee, S.H., Lee, S.T., Kim, B.J., et al., Dynamic temporal change of cerebral microbleeds: long-term follow-up MRI study, PloS One, 2011, vol. 6, no. 10. e2593. https://doi.org/10.1371/journal.pone.0025930
Akoudad, S., Ikram, M.A., Koudstaal, P.J., et al., Cerebral microbleeds are associated with the progression of ischemic vascular lesions, Cerebrovasc. Dis., 2014, vol. 37, no. 5, p. 382. https://doi.org/10.1159/000362590
Vernooij, M.W., van der Lugt, A., Ikram, M.A., et al., Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study, Neurology, 2008, vol. 70, no. 14, p. 1208. https://doi.org/10.1212/01.wnl.0000307750.41970.d9
Kim, M., Bae, H.J., Lee, J., et al., APOE epsilon2/epsilon4 polymorphism and cerebral microbleeds on gradient-echo MRI, Neurology, 2005, vol. 65, no. 9, p. 1474. https://doi.org/10.1212/01.wnl.0000183311.48144.7f
Schmidt, R., Ropele, S., Ferro, J., et al., Diffusion-weighted imaging and cognition in the leukoariosis and disability in the elderly study, Stroke, 2010, vol. 41, no. 5, p. e402. https://doi.org/10.1161/STROKEAHA.109.576629
Goos, J.D., Henneman, W.J., Sluimer, J.D., et al., Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population, Neurology, 2010, vol. 74, no. 24, p. 1954. https://doi.org/10.1212/WNL.0b013e3181e396ea
MacLullich, A.M., Wardlaw, J.M. Ferguson, K.J., et al., Enlarged perivascular spaces are associated with cognitive function in healthy elderly men, J. Neurol. Neurosurg. Psychiatry, 2004, vol. 75, no. 11, p. 1519. https://doi.org/10.1136/jnnp.2003.030858
van Swieten, J.C., Hout, J.H., van Ketel, B.A., et al., Periventricular lesions in the white matter on magnetic resonance imaging in the elderly: a morphometric correlation with arteriolosclerosis and dilated perivascular spaces, Brain, 1991, vol. 114, p. 761.
Bokura, H., Kobayashi, S., and Yamaguchi, S., Distinguishing silent lacunar infarction from enlarged Virchow—Robin spaces: a magnetic resonance imaging and pathological study, J. Neurol., 1998, vol. 245, no. 2.
Mestre, H., Kostrikov, S., and Mehta, R.I., Perivascular spaces, glymphatic dysfunction, and small vessel disease, Clin. Sci. (London), 2017, vol. 131, no. 17, p. 2257. https://doi.org/10.1042/CS20160381
Song, S.K., Sun, S.W., Ramsbottom, M.J., et al., Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water, NeuroImage, 2002, vol. 17, no. 3, p. 1429.
Pasi, M., van Uden, I.W., Tuladhar, A.M., et al., White matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease clinical consequences, Stroke, 2016, vol. 47, no. 6, p. 1679. https://doi.org/10.1161/STROKEAHA.115.012065
Hannesdottir, K., Nitkunan, A., Charlton, R.A., et al., Cognitive impairment and white matter damage in hypertension: a pilot study, Acta Neurol. Scand., 2009, vol. 119, no. 4, p. 261. https://doi.org/10.1111/j.1600-0404.2008.01098.x
Lawrence, A.J., Patel, B., Morris, R.G., et al., Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s cognition and neuroimaging in stroke (SCANS) study, PloS One, 2013, vol. 8, no. 4. e61014. https://doi.org/10.1371/journal.pone.0061014
de Groot, M., Verhaaren, B.F., de Boer, R., et al., Changes in normal-appearing white matter precede development of white matter lesions, Stroke, 2013, vol. 44, no. 4, p. 1037. https://doi.org/10.1161/STROKEAHA.112.680223
Funding
The study was not supported by any particular organization.
Conflict of interests. The authors declare the absence of a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Tarasyuk
Rights and permissions
About this article
Cite this article
Gnedovskaya, E.V., Dobrynina, L.A., Krotenkova, M.V. et al. MRI in the Assessment of Cerebral Small Vessel Disease. Hum Physiol 48, 938–945 (2022). https://doi.org/10.1134/S0362119722080023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722080023