Abstract
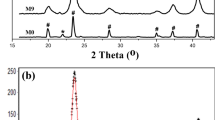
Uniform hexagonal hematite (α-Fe2O3) nanoplates were prepared via a facile alcohol-thermal reaction without using any template. Each nanoplate is enclosed by (0001) basal planes and {10\(\bar {1}\)2} side surfaces. The phase, size, shape and growth orientation of the resulting product were characterized by X-ray powder diffraction (XRD) and scanning electron microscopy (SEM), and high-resolution transmission electron microscopy (HRTEM).In order to obtain a product with more uniform and stable morphology, on the basis of predecessors' studies, the reaction time was adjusted to a small extent, so that an ideal hexagonal nanoplates was obtained. Magnetite (Fe3O4) nanoplates were obtained through a reduction process without changing the morphology and size of the resulting α-Fe2O3 nanoplates. Detection of transition from α-Fe2O3 to Fe3O4 nanoplates by X-ray photoelectron spectroscopy (XPS).The magnetic properties of these reduced nanoplates were investigated and it was found that these nanoplates have higher coercivity and lower saturation magnetization than many other nanostructured iron oxides. The surface adsorption of nonmagnetic materials and their flake morphology may be the cause of this phenomenon.






Similar content being viewed by others
REFERENCES
S. S. And and Z. Hao, J. Am. Chem. Soc. 124, 8204 (2002).
J. Park, K. An, Y. Hwang, et al., Nat. Mater. 3, 891 (2004).
B. H. Kim, N. Lee, H. Kim, et al., J. Am. Chem. Soc. 133, 12624 (2011).
X. L. Liu, Y. Yang, C. T. Ng, et al., Adv. Mater. 27, 1939 (2015).
H. M. Fan, J. B. Yi, Y. Yang, et al., ACS Nano 3, 2798 (2009).
C. Burda, X. Chen, R. Narayanan, et al., Chem. Rev. 105, 1025 (2005).
R. M. Cornell and U. Schwertmann, The Iron Oxides: Structure, Properties, Reactions, Occurences, and Uses (Wiley-VCH, Weinheim, 2003).
R. P. Cowburn, A. O. Adeyeye, and M. E. Welland, Phys. Rev. Lett. 81, 5414 (1998).
K. J. Kirk, J. N. Chapman, S. Mcvitie, et al., Appl. Phys. Lett. 75, 3683 (1999).
F. López-Urías, J. J. Torres-Heredia, and E. Muñoz-Sandoval, J. Magn. Magn. Mater. 294 (2), e7 (2005).
A. H. Lu, E. L. Salabas, and F. Schüth, Angew. Chem. 46, 1222 (2010).
Y. W. Jun, J. S. Choi, and J. Cheon, Angew. Chem. Int. Ed. 45, 3414 (2006).
C. Yang, J. Wu, and Y. Hou, Chem. Commun. 47, 5130 (2011).
J. Lu, X. Jiao, D. Chen, et al., J. Phys. Chem. C 113 (10) (2009).
L. Chen, X. Yang, C. Jian, et al., Inorg. Chem. 49, 8411 (2010).
H. Fan, M. You, et al., J. Phys. Chem. C 113, 9928 (2009).
X. Hu, J. Yu, J. Gong, et al., Adv. Mater. 19, 2324 (2010).
C. J. Jia, L. D. Sun, F. Luo, et al., J. Am. Chem. Soc. 130, 16968 (2008).
T. Fujii, F. M. F. de Groot, G. A. Sawatzky, et al., Phys. Rev. B 59, 3195 (1999).
D. Zhang, Z. Liu, S. Han, et al., Nano Lett. 4, 2151 (2004).
T. J. Daou, G. Pourroy, S. Bégin Colin, et al., Chem. Mater. 18, 4399 (2006).
D. Briggs and M. P. Seah, Anal. Chem. 61 (7) (1990).
J. Smit and H. P. J. Wijn, Ferrites: Physical Properties of Ferrimagnetic Oxides in Relation to Their Technical Applications (Philips’ Tech. Library, The Netherlands, 1959).
Kyoungja Woo, Jangwon Hong, Sungmoon Choi, et al., Chem. Mater. 16, 2814 (2004).
Funding
This work is financially supported by the National Natural Science Foundation of China (nos. 61771092, 52072245, and 51202146), the Natural Science Foundation of Shanghai (no. 17ZR1419700) and Shanghai Collaborative Innovation Center for Translational Medicine (no. TM201710, TM201810), the Liaoning Revitalization Talents Program (XLYC1907079), and Program for Natural Science Foundation of Liaoning Province (2019-ZD-0176).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, X., Zheng, H., Xue, Y. et al. Synthesis and Characterization of Regular Hexagonal Fe3O4 Nanoplates. Russ. J. Phys. Chem. 95, 1432–1438 (2021). https://doi.org/10.1134/S003602442107027X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442107027X