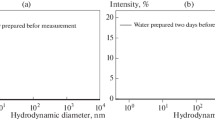
Abstract
The liquid ethylene glycol–dimethylsulfoxide system is studied by means of dynamic light scattering. Scattered light spectra and the autocorrelation functions of intensity are obtained, and relaxation times, self-diffusion coefficients, and hydrodynamic radii of associates are calculated. The results are due to solvophobic effects and micro phase separation, as is confirmed by liquid-phase separation within a certain range of temperatures and concentrations.



Similar content being viewed by others
REFERENCES
R. M. Cordeiro, S. Stirling, G. M. Fahy, and J. P. Magalhaes, Cryobiology 71, 405 (2015).
G. M. Agayan, N. K. Balabaev, and M. N. Rodnikova, Russ. J. Phys. Chem. A 95, 1283 (2021).
G. I. Egorov and D. M. Makarov, Russ. J. Phys. Chem. A 83, 693 (2009).
M. N. Rodnikova, Yu. A. Zakharova, I. A. Solonina, and D. A. Sirotkin, Russ. J. Phys. Chem. A 86, 892 (2012).
M. N. Rodnikova, Zh. Fiz. Khim. 62, 275 (1993).
E. G. Kononova, M. N. Rodnikova, I. A. Solonina, and D. A. Sirotkin, Russ. J. Phys. Chem. A 92, 1308 (2018).
I. A. Solonina, M. N. Rodnikova, M. R. Kiselev, A. V. Khoroshilov, and E. V. Shirokova, Russ. J. Phys. Chem. A 92, 918 (2018).
M. N. Rodnikova, D. B. Kayumova, Zh. V. Dobrokhotova, A. V. Khoroshilov, and T. M. Val’kovskaya, Russ. J. Phys. Chem. A 81, 1891 (2007).
F. Comelli, S. Ottani, R. Francesconi, and C. Castellari, J. Chem. Eng. Data 48, 995 (2003).
G. I. Egorov and D. M. Makarov, Russ. J. Phys. Chem. A 82, 1778 (2008).
P. Westht, J. Phys. Chem. 98, 3222 (1994).
M. N. Rodnikova, Z. Sh. Idiyatullin, J. Barthel, et al., J. Mol. Liq. 248, 898 (2017).
I. A. Solonina, M. N. Rodnikova, Yu. A. Zakharova, et al., in Proceedings of the 10th International Kurnakov Workshop on Physicochemical Analysis, Samara, 2013, Vol. 2, p. 289.
M. N. Rodnikova, J. Mol. Liq. 136, 211 (2007).
A. V. Tyurin, I. A. Solonina, M. N. Rodnikova, and D. A. Sirotkin, Russ. J. Phys. Chem. A (2021, in press).
Dynamic Light Scattering. The Method and Some Applications, Ed. by W. Brown (Clarendon, Oxford, 1993), p. 752.
C. L. Yaws, Thermophysical Properties of Chemicals and Hydrocarbons, 2nd ed. (Gulf Professional, Oxfod, 2014), p. 694.
M. Sedlak, J. Phys. Chem. B 110, 4329 (2006).
M. Sedlak, J. Phys. Chem. B 110, 4339 (2006).
M. Sedlak, J. Phys. Chem. B 110, 13976 (2006).
A. V. Orlova, T. V. Laptinskaya, N. N. Malysheva, and L. O. Kononov, J. Solution Chem. 49, 629 (2020).
G. V. Lagodzinskaya, T. V. Laptinskaya, and A. I. Kazakov, Russ. Chem. Bull. 67, 1838 (2018).
S. W. Provencher, Comput. Phys. Commun. 27, 213 (1982).
M. N. Rodnikova, L. V. Lanshina, and I. A. Chaban, Dokl. Akad. Nauk SSSR 315, 148 (1990).
I. A. Chaban, M. N. Rodnikova, L. L. Chaikov, S. V. Krivokhizha, and V. V. Zhakova, Russ. J. Phys. Chem. A 71, 1974 (1997).
M. N. Rodnikova, Acta Chim. Slov. 56, 215 (2009).
ACKNOWLEDGMENTS
The authors are grateful to Prof. L.O. Kononov for his helpful advice and discussions.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation as part of the State Assignment for the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences and with the financial support of the Russian Foundation for Basic Research (project no. 19-03-00215).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tulyabaev
APPENDIX A
APPENDIX A
Summary of concentrations (scatter angle, 40°)
[DMSO], mol % | ECF (yes or no) | ||||
|---|---|---|---|---|---|
first day | second day | third day | tenth day | later | |
0.39 | No | Low ECF | Low ECF | ECF after heating and cooling | No on the 69th day |
0.79 | No | Very low | Grew to 0.2 | ||
0.83 | No | Low ECF | Grew to 0.2 | Phase separation | |
1.11 | No | Peaks of moderate intensity | Peaks of moderate intensity | Peaks of moderate intensity | |
1.61 | Peaks of moderate intensity | Nonlinear ECF | |||
2.08 | No | No | No | Not measured | |
2.17 | Peaks of moderate intensity | Low ECF | Nonlinear ECF (0.2) | ||
2.20 | Peaks of moderate intensity | No | No | No | |
2.44 | No at Т = 303 K Low at Т = 323 K | ECF = 0.2 at Т = 323 K | ECF = 0.8 at Т = 303 K (maximum) | ECF is 0.2 at Т = 323 K | ECF remained after seven months |
2.45 | Low ECF | ECF is 0.2 | ECF > 0.2; suitable for calculations | Maximum ECF intensity | ECF remained after four months |
2.56 | No | No | No | No | |
2.72 | No | Low | Low | Low | |
3.01 | Peaks of moderate intensity | Low | Low | Low | |
3.07 | No | Low | Low | Low | |
3.61 | No | No | Low | ECF = 0.6 (appeared on eighth day) | ECF remained after 37 days; phase separation |
4.79 | Low | Low | Low | ||
5.63 | No at Т = 303 K; ECF is nonlinear at Т = 323 K (streams) | ECF = 0.6 (nonlinear) at Т = 323 K (streams) | ECF was observed and depended on angle | ECF = 0.8 | ECF remained after seven months |
6.87 | No at Т = 303 K; ECF = 0.6 (nonlinear) at Т = 323 K (streams) | ECF = 0.8 at Т = 323 K | ECF = 0.8 at Т = 303 K | Maximum ECF | ECF remained after 76 days; phase separation |
7.18 | No | No | No | Sixth day: no | |
7.93 | No | Low | Low | Low | |
8.11 | No; peaks of moderate intensity | Low | Low | Low | |
9.54 | No | No | Not measured | ||
10.95 | No | No | Not measured | ||
11.07 | No | No | Not measured | ||
12.56 | No | No | Not measured | ||
12.75 | No | No | Weak | ECF = 0.4, but peaks of moderate intensity | |
14.93 | No | Low | Low | Irregular intensity | |
15.97 | No | No | Not measured | ||
18.49 | No | Weak | ECF = 0.4 | ECF = 0.4 | ECF remained for four months |
20.07 | No | No | Not measured | ||
25.45 | No | Low | Low | Low, but grew slightly | |
26.17 | No | No | Not measured | ||
29.68 | No | No | Not measured | Similar | |
32.25 | No | Low | Low, but grew slightly | Low | |
43.74 | No | No | Not measured | ||
Rights and permissions
About this article
Cite this article
Solonina, I.A., Laptinskaya, T.V., Rodnikova, M.N. et al. Dynamic Light Scattering Study of the Liquid Ethylene Glycol–Dimethylsulfoxide System. Russ. J. Phys. Chem. 95, 1313–1319 (2021). https://doi.org/10.1134/S0036024421070244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421070244