Abstract
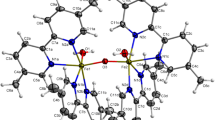
Two new neutral binuclear tetranitrosyl iron complexes of general formula [Fe2R2(NO)4] with R = 2,4-difluorothiophenyl (complex 1) and 3,4-difluorothiophenyl (complex 2), donors of nitrogen monoxide (NO), were prepared. The complexes were characterized by single-crystal X-ray diffraction, IR, Mössbauer, EPR spectroscopy, and elemental analysis. The antibacterial activity and cytotoxicity of complex 1, complex 2, and previously synthesized [\({\text{F}}{{{\text{e}}}_{{\text{2}}}}{\text{R}}_{2}^{'}\)(NO)4] with R'= 2,4-dichlorothiophenyl (complex 3) were studied for the first time. The “amount of NO–biological activity” correlations were analyzed depending on the nature and position of the substituent in the thiophenyl ligand. Complex 2 was found to have antibacterial activity that was four times as high as that of the known antibiotic kanamycin. The anti-biofilm activity of complex 2 was studied; it inhibited 46% of biofilm formation and destroyed 32% of M. Luteus biofilms, surpassing the effects of the reference drugs kanamycin and ampicillin.








Similar content being viewed by others
REFERENCES
J. T. Thomas, J. H. Robertson, and E. G. Cox, Acta Crystallogr. 11, 599 (1958). https://doi.org/10.1107/S0365110X58001602
A. R. Butler, C. Glidewell, A. R. Hyde, et al., Polyhedron 4, 797 (1985). https://doi.org/10.1016/S0277-5387(00)87029-1
A. R. Butler, C. Glidewell, A. R. Hyde, et al., Inorg. Chem. 24, 2931 (1985). https://doi.org/10.1021/ic00213a012
C. Glidewell, M. E. Harman, M. B. Hursthouse, et al., J. Chem. Res. 212–213, 1676 (1988).
T. C. Harrop, D. Song, S. J. Lippard, et al., J. Am. Chem. Soc. 128, 3528 (2006). https://doi.org/10.1021/ja060186n
C.-C. Tsou, T.-T. Lu, W.-F. Liaw, et al., J. Am. Chem. Soc. 129, 12626 (2007). https://doi.org/10.1021/ja0751375
H. M. Lee and S.-J. Chiou, Acta Crystallogr., Sect. E 65, m1600 (2009). https://doi.org/10.1107/S1600536809048065
Y.-J. Chen, W.-C. Ku, L.-T. Feng, et al., J. Am. Chem. Soc. 130, 10929 (2008). https://doi.org/10.1021/ja711494m
S.-J. Chiou, C.-C. Wang, Ch.-M. Chang, et al., J. Organomet. Chem. 693, 3582 (2008). https://doi.org/10.1016/j.jorganchem.2008.08.034
M.-C. Tsai, F.-T. Tsai, T.-T. Lu, et al., Inorg. Chem. 48, 9579 (2009). https://doi.org/10.1021/ic901675p
T.-T. Lu, H.-W. Huang, W.-F. Liaw, et al., Inorg. Chem. 48, 9027 (2009). https://doi.org/10.1021/ic9012679
R. Wang, M. A. Camacho-Fernandez, W. Xu, et al., Dalton Trans. 5, 777 (2009). https://doi.org/10.1039/B810230A
H.-H. Chang, H.-J. Huang, Y.-L. Ho, et al., Dalton Trans. 32, 6396 (2009). https://doi.org/10.1039/B902478F
T. B. Rauchfuss and T. D. Weatherill, Inorg. Chem. 21, 827 (1982). https://doi.org/10.1021/ic00132a071
M.-L. Tsai and W.-F. Liaw, Inorg. Chem. 45, 6583 (2006). https://doi.org/10.1021/ic0608849
M.-L. Tsai, C.-H. Hsieh, W.-F. Liaw, et al., Inorg. Chem. 46, 5110 (2007). https://doi.org/10.1021/ic0702567
T. C. Harrop, D. Song, and S. J. Lippard, J. Inorg. Biochem. 101, 1730 (2007). https://doi.org/10.1016/j.jinorgbio.2007.05.006
C.-H. Chen, S.-J. Chiou, H.-Y. Chen, et al., Inorg. Chem. 49, 2023 (2010). https://doi.org/10.1021/ic902324d
C.-C. Tsou and W.-F. Liaw, Chem.-Eur. J. 17, 13358 (2011). https://doi.org/10.1002/chem.201100253
W.-C. Shih, T.-T. Lu, L.-B. Yang, et al., J. Inorg. Biochem. 113, 83 (2012). https://doi.org/10.1016/j.jinorgbio.2012.03.007
C.-Y. Lu and W.-F. Liaw, Inorg. Chem. 52, 13918 (2013). https://doi.org/10.1021/ic402364p
T.-T. Lu, Y.-M. Wang, Ch.-H. Hung, et al., Inorg. Chem. 5720, 12425 (2018). https://doi.org/10.1021/acs.inorgchem.8b01818
H.-Y. Hsiao, C.-W. Chung, J. H. Santos, et al., Dalton Trans. 48, 9431 (2019). https://doi.org/10.1039/C9DT00777F
A. D. Ostrowski and P. C. Ford, Dalton Trans. 48, 10660 (2009). https://doi.org/10.1039/B912898K
A. F. Vanin, Int. J. Mol. Sci. 22, 10356 (2021). https://doi.org/10.3390/ijms221910356
Q. Xie and C. Nathan, Annu. Rev. Immunol. 15, 323 (1997). https://doi.org/10.1146/annurev.immunol.15.1.323
D. A. Wink and J. B. Mitchell, Free Radic. Biol. Med. 25, 434 (1998). https://doi.org/10.1016/S0891-5849(98)00092-6
F. Murad, Biosci. Rep. 19, 133 (1999). https://doi.org/10.1023/A:1020265417394
H.-T. Chung, H.-O. Pae, B.-M. Choi, et al., Biochem. Biophys. Res. Commun. 282, 1075 (2001). https://doi.org/10.1006/bbrc.2001.4670
K. L. Davis, E. Martin, I. V. Turko, et al., Annu. Rev. Pharmacol. Toxicol. 41, 203 (2001). https://doi.org/10.1146/annurev.pharmtox.41.1.203
D. J. Webb and I. L. Megson, Expert Opin. Investig. Drugs 11, 587 (2002). https://doi.org/10.1038/sj.bjp.0707224
D. S. Bredt, Mol. Pharmacol. 63, 1206 (2003). https://doi.org/10.1124/mol.63.6.1206
J. A. McCleverty, Chem. Rev. 104, 403 (2004). https://doi.org/10.1021/cr020623q
D. J. Singel and J. S. Stamler, Annu. Rev. Physiol. 67, 99 (2005). https://doi.org/10.1146/annurev.physiol.67.060603.090918
V. W. T. Liu and P. L. Huang, Cardiovasc. Res. 77, 19 (2008). https://doi.org/10.1016/j.cardiores.2007.06.024
D. G. Hirst and T. Robson, Curr. Pharm. Des. 16, 45 (2010). https://doi.org/10.1016/j.redox.2015.07.002
J. C. Toledo and AugustoO. Jr, Chem. Res. Toxicol. 25, 975 (2012). https://doi.org/10.1021/tx300042g
T. A. Heinrich, R. S. Silva, K. M. Miranda, et al., Br. J. Pharmacol. 169, 1417 (2013). https://doi.org/10.1111/bph.12217
S. K. Choudhari, M. Chaudhary, S. Bagde, et al., World J. Surg. Oncol. 11, 118 (2013). https://doi.org/10.1186/1477-7819-11-118
C. P. Bondonno, K. D. Croft, and J. M. Hodgson, Crit. Rev. Food Sci. Nutr. 56, 2036 (2015). https://doi.org/10.1080/10408398.2013.811212
D. Basudhar, L. A. Ridnour, R. Cheng, et al., Coord. Chem. Rev. 306, 708 (2016).https://doi.org/10.1016/j.ccr.2015.06.001
G. Herrmann and U. Graepler-Mainka, et al., Infection 44, 513 (2016). https://doi.org/10.1007/s15010-016-0879-x
L. J. Ignarro and B. A. Freeman, Nitric Oxide: Biology and Pathobiology (Elsevier, London, 2017). www.sciencedirect.com/book/9780128042731/nitric-oxide#book-info.
A. Kamm, P. Przychodzen, A. Kuban-Jankowska, et al., Nitric Oxide 93, 102 (2019). .https://doi.org/10.1016/j.niox.2019.09.005
N. Lehnert, E. Kim, H. T. Dong, et al., Chem. Rev. 121, 14682 (2021). https://doi.org/10.1021/acs.chemrev.1c00253
S. M. Aldoshin and N. A. Sanina, Fundamental Sciences—Medicine: Biophysical Medical Technologies (MAKS Press, Moscow, 2015) [in Russian].
N. A. Sanina, N. S. Emel’yanova, A. N. Chekhlov, et al., Russ. Chem. Bull. 59, 1126 (2010). https://doi.org/10.1007/s11172-010-0215-z
G. I. Kozub, T. A. Kondratieva, G. V. Shilov, et al., Russ. Chem. Bull. 72, 651 (2023). https://doi.org/10.1007/s11172-023-3829-2
N. A. Sanina, G. I. Kozub, O. S. Zhukova, et al., J. Coord. Chem. 66, 3602 (2013). https://doi.org/10.1080/00958972.2013.848980
N. A. Sanina, G. I. Kozub, T. A. Kondrat’eva, et al., J. Coord. Chem. 74, 743 (2021). https://doi.org/10.1080/00958972.2020.1869222
N. A. Sanina, G. I. Kozub, T. A. Kondrat’eva, et al., Russ. Chem. Bull. 66, 1706 (2017). https://doi.org/10.1007/s11172-017-1944-z
G. I. Kozub, N. A. Sanina, N. S. Emel’yanova, et al., Inorg. Chim. Acta 480, 132 (2018). https://doi.org/10.1016/j.ica.2018.05.015
N. A. Sanina, A. G. Krivenko, R. A. Manzhos, et al., New J. Chem. 38, 292 (2014). https://doi.org/10.1039/C3NJ00704A
N. I. Neshev, E. M. Sokolova, G. I. Kozub, et al., Russ. Chem. Bull. 69, 1987 (2020). https://doi.org/10.1007/s11172-020-2989-y
T. Stupina, A. Balakina, T. Kondrat’eva, et al., Sci. Pharm. 86, 46 (2018). https://doi.org/10.3390/scipharm86040046
V. A. Mumyatova, G. I. Kozub, T. A. Kondrat’eva, et al., Russ. Chem. Bull. 68, 1025 (2019). https://doi.org/10.1007/s11172-019-2514-3
O. V. Pokidova, V. O. Novikova, N. S. Emel’yanova, et al., Dalton Trans. 52, 2641 (2023). https://doi.org/10.1039/D2DT04047F
N. A. Sanina, S. M. Aldoshin, T. N. Rudneva, et al., Russ. J. Coord. Chem. 31, 301 (2005). https://doi.org/10.1007/s11173-005-0093-3
A. Weissberger, E. Proskauer, J. A. Riddick, et al., Organic Solvents: Phys. Properties and Methods of Purification (Interscience, New York, 1955).
G. M. Sheldrick, SHELXTL v. 6.14, Structure Determination Software Suite, 2000.
Cambridge Structural Database, version 5.43 (November, 2022).
L. J. Ignarro, J. M. Fukuto, J. M. Griscavage, et al., Proc. Natl. Acad. Sci. U.S.A. 90, 8103 (1993). https://doi.org/10.1073/pnas.90.17.8103
P. C. Ford and K. M. Miranda, Nitric Oxide 103, 31 (2020). https://doi.org/10.1016/j.niox.2020.07.004
H. H. Awad and D. M. Stanbury, Int. J. Chem. Kinet. 25, 375 (1993). https://doi.org/10.1002/kin.550250506
M. N. Möller, N. Rios, M. Trujillo, et al., J. Biol. Chem. 294, 14776 (2019). https://doi.org/10.1074/jbc.REV119.006136
N. A. Sanina, V. Sulimenkov, N. S. Emel’yanova, et al., Dalton Trans. 51, 8893 (2022). https://doi.org/10.1039/D2DT01011A
K. A. Rhodes and H. P. Schweizer, Drug Resist Updates 28, 82 (2016). https://doi.org/10.1016/j.drup.2016.07.003
C. Chan, T. C. Hardin, and J. I. Smart, Future Microbiol. 10, 1325 (2015). https://doi.org/10.2217/fmb.15.53
R. Srinivasan, S. Santhakumari, P. Poonguzhali, et al., Front. Microbiol. 12, 676458 (2021). https://doi.org/10.3389/fmicb.2021.676458
C. W. Hall and T-F. Mah, FEMS Microbiol. Rev. 41, 276 (2017). https://doi.org/10.1093/femsre/fux010
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Government Assignment no. АААА-А19-119071890015-6).
Author information
Authors and Affiliations
Contributions
N.A. Sanina came up with and designed an experiment. A.S. Konyukhova synthesized samples and studied their NO-donor activity. D.V. Korchagin and S.M. Aldoshin carried out single-crystal X-ray diffraction experiments. N.S. Ovanesyan studied samples by Mössbauer spectroscopy. A.V. Kulikov performed EPR spectroscopic studies. V.A. Mumyatova and A.A. Terent’ev studied the antibacterial and cytotoxic activities of the compounds. All authors discussed the results.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Supplementary Information










Rights and permissions
About this article
Cite this article
Sanina, N.A., Konyukhova, A.S., Korchagin, D.V. et al. Effect of F Substituents in Thiophenol on the Structure and Properties of µ2-S-(Difluorothiolate)tetranitrosyl Iron Binuclear Complexes. Russ. J. Inorg. Chem. 68, 1143–1158 (2023). https://doi.org/10.1134/S0036023623601526
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623601526