Abstract
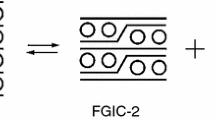
The reduction of [(nacnac)MnCl]2 (1) (nacnac = HC(C(Me)N(2.6-i-Pr2C6H3))2) by potassium intercalated graphite KC8 in toluene and benzene is studied. It is established that in both cases, Mn(II) reduces to Mn(I), giving [(nacnac)Mn]2 dimer (2) with the Mn–Mn bond. In addition, solvent interaction products form. In the first case, the product of toluene meta-deprotonation [(nacnac)Mn(m-C6H4CH3)] (3) forms; in the second, the product of benzene reduction [{Mn(nacnac)}2(μ–η4:η4-C6H6)] C6H6 (4) forms. The structures of complexes 3 and 4 are determined by single crystal X-ray diffraction (XRD). A set of crystalline reaction products and their ratio is determined by quantitative powder XRD using the Rietveld method: in the case of toluene, 2, 3, and [(nacnac)MnH]2 are in a 1:1:4 ratio; in the case of benzene, 2, 4·C6H6, and 1 are in a 3:8.5:1 ratio, and also trace amounts of a crystalline phase with an unknown structure.






Similar content being viewed by others
REFERENCES
R. L. Webster. Dalton Trans., 2017, 46, 4483-4498. https://doi.org/10.1039/C7DT00319F
S. F. McWilliams, D. L. J. Broere, C. J. V. Halliday, S. M. Bhutto, B. Q. Mercado, and P. L. Holland. Nature, 2020, 584, 221-226. https://doi.org/10.1038/s41586-020-2565-5
I. Fairlamb and J. Lynam. Organometallic Chemistry, Vol. 39. Cambridge, UK: The Royal Society of Chemistry, 2014. https://doi.org/10.1039/9781849737692
S. Hohloch, B. M. Kriegel, R. G. Bergman, and J. Arnold. Dalton Trans., 2016, 45, 15725-15745. https://doi.org/
.1039/B715027J
J. M. Smith, A. R. Sadique, T. R. Cundari, K. R. Rodgers, G. Lukat-Rodgers, R. J. Lachicotte, C. J. Flaschenriem,J. Vela, and P. L. Holland. J. Am. Chem. Soc., 2006, 128, 756-769. https://doi.org/10.1021/ja052707x
W. H. Monillas, G. P. A. Yap, and K. H. Theopold. Angew. Chem., Int. Ed., 2007, 46, 6692-6694. https://doi.org/
Y.-C. Tsai, P.-Y. Wang, K.-M. Lin, S.-A. Chen, and J.-M. Chen. Chem. Commun., 2008, 205-207. https://doi.org/
Y.-C. Tsai, P.-Y. Wang, S.-A. Chen, and J.-M. Chen. J. Am. Chem. Soc., 2007, 129, 8066/8067. https://doi.org/
D. J. E. Spencer, N. W. Aboelella, A. M. Reynolds, P. L. Holland, and W. B. Tolman. J. Am. Chem. Soc., 2002, 124, 2108/2109. https://doi.org/10.1021/ja017820b
K.-C. Chang, C.-F. Lu, P.-Y. Wang, D.-Y. Lu, H.-Z. Chen, T.-S. Kuo, and Y.-C. Tsai. Dalton Trans., 2011, 40, 2324-2331. https://doi.org/10.1039/C0DT01061H
J. Chai, H. Zhu, A. C. Stückl, H. W. Roesky, J. Magull, A. Bencini, A. Caneschi, and D. Gatteschi. J. Am. Chem. Soc., 2005, 127, 9201-9206. https://doi.org/10.1021/ja042269e
Y. Wang, B. Quillian, P. Wei, H. Wang, X.-J. Yang, Y. Xie, R. B. King, P.v.R. Schleyer, H. F. Schaefer, andG. H. Robinson. J. Am. Chem. Soc., 2005, 127, 11944/11945. https://doi.org/10.1021/ja053819r
D. J. Webb, C. M. Fitchett, M. Lein, and J. R. Fulton. Chem. Commun., 2018, 54, 460-462. https://doi.org/
F. Spitzer, C. Graßl, G. Balázs, E. M. Zolnhofer, K. Meyer, and M. Scheer. Angew. Chem., Int. Ed., 2016, 55, 4340-4344. https://doi.org/10.1002/anie.201510716
F. Spitzer, C. Graßl, G. Balázs, E. Mädl, M. Keilwerth, E. M. Zolnhofer, K. Meyer, and M. Scheer. Chem. – Eur. J., 2017, 23, 2716-2721. https://doi.org/10.1002/chem.201605451
D. M. Roundhill. Photochemistry and Photophysics of Metal Complexes. Boston, MA: Springer, 1994. https://doi.org/10.1007/978-1-4899-1495-8
T. K. Mukhopadhyay, M. Flores, T. L. Groy, and R. J. Trovitch. Chem. Sci., 2018, 9, 7673-7680. https://doi.org/
T. T. Nguyen, J.-H. Kim, S. Kim, C. Oh, M. Flores, T. L. Groy, M.-H. Baik, and R. J. Trovitch. Chem. Commun., 2020, 56, 3959-3962. https://doi.org/10.1039/C9CC09921B
Apex3 software suite: Apex3, SADABS-2016/2 and SAINT, version 2018.7-2. Madison, WI: Bruker AXS Inc., 2017.
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3-8. https://doi.org/10.1107/S2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339-341. https://doi.org/10.1107/S0021889808042726
A. V. Alexeev and S. A. Gromilov. J. Struct. Chem., 2010, 51(4), 744-757. https://doi.org/10.1007/s10947-010-0110-3
A. V. Alexeev and S. A. Gromilov. J. Struct. Chem., 2010, 51(1), 156-165. https://doi.org/10.1007/s10947-010-0022-2
C. Prescher and V. B. Prakapenka. High Press. Res., 2015, 35, 223-230. https://doi.org/10.1080/08957959.
.1059835
J. Chai, H. Zhu, H. Fan, H. W. Roesky, and J. Magull. Organometallics, 2004, 23, 1177-1179. https://doi.org/
D. R. Armstrong, J. García-Álvarez, D. V. Graham, G. W. Honeyman, E. Hevia, A. R. Kennedy, and R. E. Mulvey. Chem. – Eur. J., 2009, 15, 3800-3807. https://doi.org/10.1002/chem.200801928
L. Dahlenburg and K.-M. Frosin. Chem. Ber., 1988, 121, 865-869. https://doi.org/10.1002/cber.19881210509
M.A. Esteruelas, M. Oliván, A. Vélez. Organometallics, 2015, 34, 1911–1924. https://doi.org/10.1021/
E. Nicolas, X.-F. le Goff, S. Bouchonnet, and N. Mézailles. Chem. Commun., 2012, 48, 8350. https://doi.org/
D. D. L. Jones, I. Douair, L. Maron, and C. Jones. Angew. Chem., Int. Ed., 2021, 60, 7087-7092. https://doi.org/
C. Ni, B. D. Ellis, J. C. Fettinger, G. J. Long, and P. P. Power. Chem. Commun., 2008, 1014-1016. https://doi.org/
F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen, and R. Taylor. J. Chem. Soc. Perkin Trans. 2, 1987, S1. https://doi.org/10.1039/p298700000s1
S. Yao, T. Szilvási, N. Lindenmaier, Y. Xiong, S. Inoue, M. Adelhardt, J. Sutter, K. Meyer, and M. Driess. Chem. Commun., 2015, 51, 6153-6156. https://doi.org/10.1039/C5CC00147A
X. Dai, P. Kapoor, and T. H. Warren. J. Am. Chem. Soc., 2004, 126, 4798/4799. https://doi.org/10.1021/
A. Hicken, A. J. P. White, and M. R. Crimmin. Inorg. Chem., 2017, 56, 8669-8682. https://doi.org/10.1021/
Y. M. Badiei, A. Dinescu, X. Dai, R. M. Palomino, F. W. Heinemann, T. R. Cundari, and T. H. Warren. Angew. Chem., Int. Ed., 2008, 47, 9961-9964. https://doi.org/10.1002/anie.200804304
A. D. Phillips, G. Laurenczy, R. Scopelliti, and P. J. Dyson. Organometallics, 2007, 26, 1120-1122. https://doi.org/10.1021/om070017r
A. Moreno, P. S. Pregosin, G. Laurenczy, A. D. Phillips, and P. J. Dyson. Organometallics, 2009, 28, 6432-6441. https://doi.org/10.1021/om900634s
P. H. M. Budzelaar, N. N. P. Moonen, R. de Gelder, J. M. M. Smits, and A. W. Gal. Chem. – Eur. J., 2000, 6, 2740-2747. https://doi.org/10.1002/1521-3765(20000804)6:15%3C2740::AID-CHEM2740%3E3.0.CO;2-0
T. L. Gianetti, G. Nocton, S. G. Minasian, N. C. Tomson, A. L. D. Kilcoyne, S. A. Kozimor, D. K. Shuh, T. Tyliszczak, R. G. Bergman, and J. Arnold. J. Am. Chem. Soc., 2013, 135, 3224-3236. https://doi.org/10.1021/ja311966h
G. Bai, P. Wei, and D. W. Stephan. Organometallics, 2005, 24, 5901-5908. https://doi.org/10.1021/om050544f
C. M. Kotyk, M. E. Fieser, C. T. Palumbo, J. W. Ziller, L. E. Darago, J. R. Long, F. Furche, and W. J. Evans. Chem. Sci., 2015, 6, 7267-7273. https://doi.org/10.1039/C5SC02486B
N. Reinfandt, N. Michenfelder, C. Schoo, R. Yadav, S. Reichl, S. N. Konchenko, A. N. Unterreiner, M. Scheer, and P. W. Roesky. Chem. – Eur. J., 2021, 27, 7862-7871. https://doi.org/10.1002/chem.202100605
Y.-Z. Ma, N. A. Pushkarevsky, T. S. Sukhikh, A. E. Galashov, A. G. Makarov, P. W. Roesky, and S. N. Konchenko. Eur. J. Inorg. Chem., 2018, 3388-3396. https://doi.org/10.1002/ejic.201800201
B. H. Toby and R. B. Von Dreele. J. Appl. Crystallogr., 2013, 46, 544-549. https://doi.org/10.1107/S002188
Funding
The work was supported by the Russian Science Foundation (Grant No. 19-73-00183) and the Ministry of Science and Higher Education of the Russian Federation (Projects Nos. 121031700321-3, 121031700313-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 10, pp. 1684-1691.https://doi.org/10.26902/JSC_id80783
Rights and permissions
About this article
Cite this article
Afonin, M.Y., Sedelnikova, A.Y., Konokhova, A.Y. et al. STRUCTURE AND COMPOSITION OF [(nacnac)MnCl]2 (nacnac = HC(C(Me)N(2.6-i-Pr2C6H3))2) PRODUCTS REDUCED BY POTASSIUM-INTERCALATED GRAPHITE IN TOLUENE AND BENZENE. J Struct Chem 62, 1580–1587 (2021). https://doi.org/10.1134/S0022476621100139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621100139