Abstract
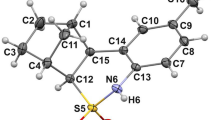
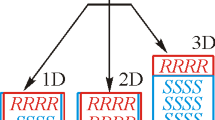
The crystal structures of four representatives of sulfinamides of the thiazine series are comparatively analyzed for the occurrence of different supramolecular associates, inter- and intramolecular interactions. With a similar molecular geometry, different packing motifs occur in the crystals yielding different-type supramolecular associates due to classical N–H⋯O hydrogen bonds. The features of transferring the sulfinamide open-chain supramolecular synthon from racemic to homochiral environment consisting in the appearance of “secondary cross-linking” are studied.







Similar content being viewed by others
REFERENCES
G. M. J. Schmidt. Pure Appl. Chem., 1971, 27, 647.
V. R. Thalladi, B. S. Goud, V. -J. Hoy, F. H. Allen, J. A. K. Howard, and G. R. Desiraju. Chem. Commun., 1996, 3, 401.
J. V. Barth, G. Constantini, and K. Kern. Nature, 2005, 437, 671.
C. A. Palma, M. Bonini, T. Breiner, and P. Samori. Adv. Mater., 2009, 21, 1383.
J. A. A. W. Elemans, S. B. Lei, and S. De Feyter. Angew. Chem., Int. Ed., 2009, 48, 7298.
J. Simon and P. Bassoul. Design of Molecular Materials: Supramolecular Engineering. Wiley-VCH, 2000.
A. Ciesielski, C.A. Palma, M. Bonini, and P. Samori. Adv. Mater., 2010, 22, 3506.
G. R. Desiraju. Angew. Chem., Int. Ed., 1995, 34, 2311.
G. R. Desiraju. Crystal Engineering: The Design of Organic Solids. Elsevier: Amsterdam, 1989.
D. S. Reddy, D. C. Craig, and G. R. Desiraju. J. Am. Chem. Soc., 1996, 118, 4090.
V. R. Thalladi, B. S. Goud, V. J. Hoy, F. H. Allen, J. A. K. Howard, and G. R. Desiraju. Chem. Commun., 1996, 401.
O. A. Lodochnikova, L. Z. Latypova, T. I. Madzhidov, G. A. Chmutova, J. K. Voronina, A. T. Gubaidullin, and A. R. Kurbangalieva. CrystEngComm, 2019, 21, 1499.
O. N. Kataeva, K. E. Metlushka, Z. R. Yamaleeva, K. A. Ivshin, A. G. Kiiamov, O. A. Lodochnikova, K. A. Nikitina, D. N. Sadkova, L. N. Punegova, A. D. Voloshina, A. P. Lyubina, A. S. Sapunova, O. G. Sinyashin, and V. A. Alfonsov. Cryst. Growth Des., 2019, 19, 4044.
O. A. Lodochnikova, D. B. Krivolapov, V. A. Startseva, L. E. Nikitina, A. V. Bodrov, N. P. Artemova, V. V. Klochkov, T. I. Madzhidov, G. A. Chmutova, and I. A. Litvinov. Phosphorus, Sulfur Silicon Relat. Elem., 2015, 190, 2222.
O. A. Lodochnikova, A. R. Zaripova, R. R. Fayzullin, A. I. Samigullina, I. I. Vandyukova, L. N. Potapova, and A. R. Kurbangalieva. CrystEngComm, 2018, 20, 3218.
D. P. Gerasimova, A. F. Saifina, D. V. Zakharychev, A. R. Zaripova, R. R. Fayzullin, A. R. Kurbangalieva, and O. A. Lodochnikova. J. Struct. Chem., 2021, 62(5), 727-739, DOI: 10.1134/S0022476621050097.
R. R. Fayzullin, S. A. Shteingolts, O. A. Lodochnikova, V. L. Mamedova, D. E. Korshin, and V. A. Mamedov. CrystEngComm, 2019, 21, 1587.
I. V. Fedyanin and K. A. Lyssenko. CrystEngComm, 2013, 15, 10086.
APEX (Version 2.1), SAINTPlus, Data Reduction and Correction Program Version 7.31A. Bruker AXS: Madison, Wisconsin, USA, 2006.
G. M. Sheldrick. SADABS. University of Göttingen: Göttingen, Germany, 2004.
G. M. Sheldrick. SHELXL-97 Program for Crystal Structure Refinement. University of Göttingen: Germany, 1997.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112.
L. J. Farrugia. J. Appl. Crystallogr., 1999, 32, 837.
R. Dovesi, A. Erba, R. Orlando, C. M. Zicovich-Wilson, B. Civalleri, L. Maschio, M. Rérat, S. Casassa, J. Baima, S. Salustro, and B. Kirtman. Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1360.
C. Gatti, V. R. Saunders, and C. Roetti. J. Chem. Phys., 1994, 101, 10686.
C. Gatti and S. Casassa. TOPOND14 Users Manual. 2017, https://www.crystal.unito.it/topond/topond.pdf.
Ya. V. Veremeichik, P. V. Merabov, V. V. Plemenkov, O. A. Lodochnikova, D. B. Krivolapov, I. A. Litvinov, L. V. Spirikhin, and A. N. Lobov. Russ. J. Gen. Chem., 2012, 82, 1416.
Ya. V. Veremeichik, D. N. Shurpik, O. A. Lodochnikova, and V. V. Plemenkov. Russ. J. Org. Chem., 2016, 52, 92.
Ya. V. Veremeichik, D. N. Shurpik, O. A. Lodochnikova, and V. V. Plemenkov. Russ. J. Gen. Chem., 2016, 86, 296.
Ya. V. Veremeichik, P. V. Merabov, A. V. Chuiko, V. V. Plemenkov, and O. A. Lodochnikova. Russ. J. Org. Chem., 2013, 49, 1605.
E. V. Mironova, O. A. Lodochnikova, D. B. Krivolapov, I. A. Litvinov, Y. V. Veremeichik, and V. V. Plemenkov. J. Struct. Chem., 2014, 55(3), 539.
F. Dufour, C. Gervais, M. N. Petit, G. Perez, and G. Coquerel. J. Chem. Soc., Perk. Trans. 2, 2001, 10, 2022.
V. Y. Torbeev, K. A. Lyssenko, O. N. Kharybin, M. Y. Antipin, and R. G. Kostyanovsky. J. Phys. Chem., 2003, 107, 13523.
M. Iwaoka, S. Takemoto, and S. Tomoda. J. Amer. Chem. Soc., 2002, 124, 10613.
E. Espinosa, E. Mollins, and C. Lecomte. Chem. Phys. Lett., 1998, 285, 170.
S. A. Shteingolts and R. R. Fayzullin. Cryst. Growth Des., 2020, 20, 2074.
S. A. Shteingolts, A. F. Saifina, L. F. Saifina, V. E. Semenov, G. K. Fukin, and R. R. Fayzullin. J. Mol. Struct., 2021, 1228, 129724.
I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson, and R. Taylor. Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389.
Funding
The work was supported by RSF grant 17-13-01209. The synthetic part of the work was supported by the State Competitiveness Program for the Kazan Federal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2021, published in Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 6, pp. 1043-1052.https://doi.org/10.26902/JSC_id74451
Rights and permissions
About this article
Cite this article
Gerasimova, D.P., Plemenkov, V.V. & Lodochnikova, O.A. CRYSTAL STRUCTURE OF SULFINAMIDES OF THE THIAZINE SERIES: FEATURES OF TRANSFERRING THE OPEN-CHAIN SUPRAMOLECULAR SYNTHON FROM THE RACEMIC TO HOMOCHIRAL ENVIRONMENT. J Struct Chem 62, 974–983 (2021). https://doi.org/10.1134/S0022476621060172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621060172