Abstract
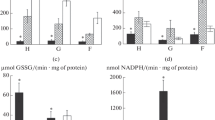
Tissue- and species-specific features of superoxide dismutase (SOD) and catalase activity, as well as levels of thiobarbituric acid reactive substances (TBARS), were investigated in the hepatopancreas, gills and foot of the three Black Sea bivalve mollusks—lagoon cockle Cerastoderma glaucum, mussel Mytilus galloprovincialis and blood clam Anadara kagoshimensis—sharing high natural oxidative stress tolerance. Cockle tissues displayed a far lower TBARS level compared to that in the mussel and blood clam, with values increasing in the following sequence: cockle–clam–mussel. Of the three bivalve species, a highest activity of both antioxidant enzymes was detected in all cockle tissues. The blood clam and lagoon cockle, as burrowing mollusks, shared a similar tissue specificity and displayed a highest activity of both enzymes in the gills. The blood clam was appreciably superior to the mussel as to SOD and catalase activity in the gills but inferior in terms of SOD activity in the hepatopancreas and foot. The revealed features reflect a different oxidative stress tolerance of the bivalves studied in their natural habitats. A highest oxidative stress sensitivity was found in the mussel. By contrast, the lagoon cockle was distinguished by a highest oxidative stress tolerance, while the blood clam occupied an intermediate position. These species-specific features enable mollusks to successfully adapt to oxidative stress which typically occurs in benthic biotopes of the Black Sea.



Similar content being viewed by others
REFERENCES
Agarkov, A.A., Popova, T.N., and Mataso-va, L.V., Effect of melatonin on the antioxidant status in type 2 diabetes mellitus in rats, Biomed. Khim., 2013, vol. 59(4), pp. 434–442.
Alyomov, S.V., The current state of macrozoobenthos in the Sevastopol Bay as determined by benthic survey of 1997, Ekol. Morya, 1999, vol. 48, pp. 73–75.
Girin, S.V., Modification of the method for assaying catalase activity in biological substrates, Lab. Diagnost., 1999, vol. 4, pp. 45–46.
Goromosova, S.A. and Shapiro, A.Z., Osnovnye cherty biokhimii energeticheskogo obmena midii (Basic Biochemical Characteristics of Energy Metabolism in Mussels), Moscow, 1984.
Istomina, A.A., Dovzhenko, N.V., Belcheva, N.N., and Chelomin, V.P., Activity of antioxidant enzymes in different mollusk species under conditions of hypoxia/anoxia, Izv. Samar. Nauch. Ts. RAN, 2011, vol. 13, no. 1(5), pp. 1106–1108.
Klimova, Ya.S. and Chuiko, G.M., Antioxidant status of bivalve mollusks Dreissena polymorpha and D. bugensis (Dreissenidae, Bivalvia) from the Volga reach of the Rybinsk reservoir, Povolzh. Ekol. Zh., 2015, no. 1, pp. 33–41.
Kolyuchkina, G.A. and Ismailov, A.D., Parameters of extrapallial fluid in bivalve mollusks: nonspecific biomarkers of transient pollution of water environment, Okeanol., 2007, vol. 47, no. 2, pp. 233–240.
Malakhova, L.V., Kostova, S.K., and Plotitsy-na, O.V., Chemical pollution of components of the Kazachya Bay ecosystem (Black Sea),
Khimicheskoe zagryaznenie komponentov ekosistemy Kazachyei bukhty (Chernoe more) (Environmental safety of coastal and offshore zones and complex use of offshore resources), Sevastopol, 2003, iss. 9, pp. 112–116.
Mironov, O.G., Kiryukhina, L.N., and Alemov, S.V., Ecological characterization of the Kazachya Bay (Black Sea), Ekol. Morya, 2002, iss. 61, pp. 85–89.
Mikhailova, T.V., Reproductive features of Cerastoderma glaucum (Mollusca, Bivalvia) in the Black Sea, Ekol. Morya, 1986, vol. 23, pp. 64–68.
Pereslegina, I.A., Activity of antioxidant enzymes in saliva of healthy children, Lab. Delo, 1989, no. 11, pp. 20–23.
Revkov, N.K., Macrozoobenthos of the Ukranian Black Sea shelf, Promyslovie bioresursy Chernogo i Azovskogo morei (Commercial bioresources of the Black and Azov Seas), Eremeev, V.N., Gaevskaya, A.V., Shulman, G.E., and Zagorodnyaya, Yu.A., Eds., Sevastopol, 2011, ch. 5.1, pp. 140–162.
Soldatov, A.A., Aleksandrova, O.L., Golovi-na, I.V., and Stolbov, A.Ya., Enzymatic system of antioxidant defense in the Black Sea mollusk Mytilus galloprovincialis Lam. with pigmented and depigmented tissue structures, Dokl. NAN Ukr., 2003, no. 5, pp. 162–167.
Fokina, N.N., Nefedova, Z.A., and Nemo-va, N.N., Biochemical adaptations of marine bivalves to hypoxia: a review, Trudy Karel. NTs RAN, 2011, no. 3, pp. 121–130.
Kholodov, V.I., Pirkova, A.V., and Ladygi-na, L.V., Vyrashchivanie midii i ustrits v Chernom more (Mussel and oyster farming in the Black Sea), Eremeev, V.N., Ed., Sevastopol, 2010.
Chikina, M.V., Klyuchkina, G.A., and Kucheruk, N.V., Aspects of reproductive biology of Scapharca inaequivalvis (Bruguiere) (Bivalvia, Arcidae) in the Black Sea, Ekol. Morya, 2003, iss. 64, pp. 72–77.
Baudrimont, M., Schäfer, J., Marie, V., Maury-Brachet, R., Bossy, C., Boudou, A., and Blanc, G., Geochemical survey and metal bioaccumulation of three bivalve species (Crassostrea gigas, Cerastoderma edule and Ruditapes philippinarum) in the Nord Medoc salt marshes (Gironde estuary, France), Sci. Tot. Environ., 2005. vol. 337, no. 1–3, pp. 265–280.
Box, A., Sureda, A., Galgani, F., Pons, A., and Deudero, S., Assessment of environmental pollution at Balearic Islands applying oxidative stress biomarkers in the mussel Mytilus galloprovincialis, Comp. Biochem. Physiol. C, Pharmacol. Toxicol., 2007, vol. 146, no. 4, pp. 531–539.
Boyden, C.R., The behaviour, survival and respiration of the cockles Cerastoderma edule and C. glaucum in air, J. Marine Biol. Ass. UK, Cambridge Core, 1972, vol. 52, no. 3, pp. 661–680.
Carroll, J.L., Strategies of anaerobiosis in New Zealand in faunal bivalves: adaptations to environmental and functional hypoxia, N. Z. J. Mar. Freshwater Res., 1995, vol. 29, pp. 137–146.
Gostyukhina, O.L., Comparative characteristics of the antioxidant glutathione complex in the Black Sea molluscs Mytilus galloprovincialis Lam. and Anadara inaequivalvis Br., J. Evol. Biochem. Physiol., 2013, vol. 49, no. 1, pp. 59–65.
Gostiukhina, O.L., Soldatov, A.A., Golovi-na, I.V., and Borodina, A.V., Content of carotenoids and the state of tissue antioxidant enzymatic complex in bivalve mollusc Anadara inaequivalvis Br., J. Evol. Biochem. Physiol., 2013, vol. 49, 3, pp. 309–315.
Gostiukhina, O.L. and Golovina, I.V., Comparative analysis of antioxidant complex of the Black Sea mollusks Mytilus galloprovincialis, Anadara inaequivalvis and Crassostrea gigas, Hydrobiol. J., 2013, vol. 49, no. 3, pp. 77–84.
Gostyukhina, O.L. and Andreenko, T.I., Enzymatic and low-molecular-weight units of antioxidant complex in two species of the Black Sea mollusks with different resistance to oxidative stress: Mytilus galloprovincialis Lam. and Anadara kagoshimensis (Tokunaga, 1906), Zh. Obshch. Biol., 2018, vol. 79, no. 6, pp. 483–493.
Freitas, R., Costa, E., Velez, C., Santos, J., Lima, A., Oliveira, C., Rodrigues, A., Quintino, V., and Figueira, E., Looking for suitable biomarkers in benthic macroinvertebrates inhabiting coastal areas with low metal contamination: Comparison between the bivalve Cerastoderma edule and the polychaete Diopatra neapolitana, Ecotoxicol. Environ. Saf., 2012, vol. 75, pp. 109–118.
Leontarakis, P.K., Loukia, I., Xatzianasta-siou, L.I., and Theodorou, J.A., Biological aspects of the lagoon cockle, Cerastoderma glaucum (Poiret, 1879) in a coastal lagoon in Keramoti, Greece in the Northeastern Mediterranean, J. Shellfish Res., 2008, vol. 27, no. 5, pp. 1171–1175.
Livingstone, D.R., Origins and evolution of pathways of anaerobic metabolism in the animal kingdom, Amer. Zoologist, 1991, vol. 31, pp. 522–534.
Livingstone, D.R., Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms, Mar. Pollut. Bull., 2001, vol. 42, no. 8, pp. 656–666.
Lowry, O.H., Rosebrough, N.J., and Farr, A.L., Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, vol. 193, no. 266, p. 75.
Maisano, M., Cappello, T., Natalotto, A., Vitale, V., Parrino, V., Giannetto, A., Oliva, S., Mancini, G., Cappello, S., Mauceri, A., and Fasulo, S., Effects of petrochemical contamination on caged marine mussels using a multi-biomarker approach: histological changes, neurotoxicity and hypoxic stress, Mar. Environ. Res., 2017, vol. 128, pp. 114–123.
Manduzio, H., Rocher, B., Durand, F., Galap, C., and Leboulenge, F., The point about oxidative stress in mollusks (Review), ISJ, 2005, vol. 91, no. 2, pp. 91–104.
Marques, A., Pilo, D., Araujo, O., Pereira, F., Guilherme, S., Carvalho, S., Santos, A.M., Pacheco, M., and Pereira, P., Propensity to metal accumulation and oxidative stress responses of two benthic species (Cerastoderma edule and Nephtys hombergii): are tolerance processes limiting their responsiveness? Ecotoxicol., 2016, vol. 25, no. 4, pp. 664–676.
Mejdoub, Z., Fahde, A., Loutfi, M., and Kabi-ne, M., Oxidative stress responses of the mussel Mytilus galloprovincialis exposed to emissary's pollution in coastal areas of Casablanca, Ocean and Coastal Management, 2017, vol. 136, pp. 95–103.
Miyamoto, Y. and Iwanaga, C., Effects of sulphide on anoxia-driven mortality and anaerobic metabolism in the ark shell
Anadara kagoshimensis, J. Marine Biol. Ass. UK, 2017, vol. 97, no. 2, pp. 329–336.
Ohkawa, H., Ohishi, N., and Yagi, K., Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction, Analyt. Biochem., 1979, vol. 95, no. 1, pp. 351–358.
Regoli, F. and Giuliani, M., Oxidative pathways of toxicity and oxidative stress biomarkers in marine organisms, Mar. Environ. Res., 2014, vol. 93, pp. 106–117.
Shapiro, A.Z., On the content of macroergic compounds in mussel tissues under normal and hypoxic conditions, J. Biol. Mor., 1981, no. 2, pp. 69–75.
Soldatov, A.A., Gostyukhina, O.L., and Golovi-na, I.V., State of the antioxidant enzyme complex in tissues of the Black Sea mollusc Mytilus galloprovincialis under natural oxidative stress, J. Evol. Biochem. Physiol., 2008, vol. 44, no. 2, pp. 175–182.
Trevisan, R., Mello, D., Delapedra, G., Silva, D., Arl, M., Danielli, N., and Dafre, A., Gills as a glutathione-dependent metabolic barrier in Pacific oysters Crassostrea gigas: absorption, metabolism and excretion of a model electrophile, Aquat. Toxicol., 2016, vol. 173, pp. 105–119.
Valavanidis, A., Vlahogianni, T., Dassenakis, M., and Scoullos, M., Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants, Ecotoxicol. Environ. Safety, 2006, vol. 64, no. 2, pp. 178–189.
Velez, C., Pires, A., Leandro, S., Cardoso, P., Moreira, A., Figueira, E., Soares, A., and Freitas, R., The use of Cerastoderma glaucum as a sentinel and bioindicator species: take-home message, Ecol. Indicators, 2016, vol. 62, pp. 228–241.
Vlahogianni, T., Dassenakis, M., Scoullos, M.J., and Valavanidis, A., Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos Gulf of Greece, Mar. Pollut. Bull., 2007, vol. 54, no. 9, pp. 1361–1371.
Zwaan, A., Cortesi, P., ThiHart, G., van den Roos, J., and Storey, K.B., Differential sensitivities to hypoxia by two anoxia-tolerant marine molluscs: a biochemical analysis, Mar. Biol., 1991, vol. 111, pp. 343–351.
ACKNOWLEDGMENTS
We are sincerely grateful to A.V. Borodina and A.Yu. Gostyukhin for the aid in collecting mollusks and to N.V. Nikolsky for his consultation on statistics.
Funding
This work was implemented within a state assignment to A.O. Kovalevsky Institute of Biology of the Southern Seas (Sevastopol) (АААА-А18-118021490093-4 of 14.02.2018 and АААА-А19-119060690014-5 of 06.06.2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All applicable international, national and institutional principles of handling and using experimental animals for scientific purposes were observed.
This study did not involve human subjects as research objects.
Rights and permissions
About this article
Cite this article
Gostuykhina, O.L., Andreenko, T.I. Superoxide Dismutase and Catalase Activities in Tissues of the Black Sea Bivalve Mollusks Cerastoderma glaucum (Bruguière, 1789), Anadara kagoshimensis (Tokunaga, 1906) and Mytilus galloprovincialis Lam. as Related to Adaptation to Their Habitats. J Evol Biochem Phys 56, 113–124 (2020). https://doi.org/10.1134/S0022093020020039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093020020039