Abstract—
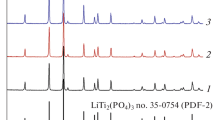
The processes occurring during the solid-state synthesis of germanium-doped lithium titanium phosphate have been studied. The formation of LiTi2 – xGex(PO4)3 has been shown to proceed through the titanium pyrophosphate formation followed by its transformation into materials with the NASICON structure. The process is completed at 1073 K. To produce ceramics with an optimal conductivity, annealing at 1173 K is required. Based on the results obtained, a two-stage synthesis procedure was developed. The highest ionic conductivity (3.9 × 10–5 Ohm–1 cm–1 at 433 K) and the lowest activation energy (46 ± 1 kJ/mol) were observed for LiTi2 – xGex(PO4)3 materials with a titanium substitution degree of 20–25% (x = 0.4–0.5). It can be attributed to an optimal size of lithium transport channels.







Similar content being viewed by others
REFERENCES
Lu, L., Han, X., Li, J., Hua, J., and Ouyang, M., A review on the key issues for lithium-ion battery management in electric vehicles, J. Power Sources, 2013, vol. 226, pp. 272–288.
Nitta, N., Wu, F., Lee, J.T., and Yushin, G., Li-ion battery materials: present and future, Mater. Today, 2015, vol. 18, pp. 252–264.
Yaroslavtsev, A.B., Stenina, I.A., and Golubenko, D.V., Membrane materials for energy production and storage, Pure Appl. Chem., 2020, vol. 92, no. 7, pp. 1147–1157.
Xu, K., Electrolytes and interphases in Li-ion batteries and beyond, Chem. Rev., 2014, vol. 114, pp. 11503–11618.
Quartarone, E. and Mustarelli, P., Review-emerging trends in the design of electrolytes for lithium and post-lithium batteries, J. Electrochem. Soc., 2020, vol. 167, paper 050508.
Stenina, I.A. and Yaroslavtsev, A.B., Nanomaterials for lithium-ion batteries and hydrogen energy, Pure Appl. Chem., 2017, vol. 89, no. 8, pp. 1185–1194.
Li, Q., Chen, J., Fan, L., Kong, X., and Lu, Y., Progress in electrolytes for rechargeable Li-based batteries and beyond, Green Energy Environ., 2016, vol. 1, pp. 18–42.
Bushkova, O.V., Yaroslavtseva, T.V., and Dobrovolsky, Yu.A., New lithium salts in electrolytes for lithium-ion batteries (review), Russ. J. Electrochem., 2017, vol. 53, pp. 677–699.
Braga, M.H., Subramaniyam, C., Murchison, A.J., and Goodenough, J.B., Nontraditional, safe, high voltage rechargeable cells of long cycle life, J. Am. Chem. Soc., 2018, vol. 140, pp. 6343–6352.
Goodenough, J.B. and Braga, M.H., Batteries for electric road vehicles, Dalton Trans., 2018, vol. 47, pp. 645–648.
Yue, L., Ma, J., Zhang, J., Zhao, J., and Chen, L., All solid-state polymer electrolytes for high-performance lithium ion batteries, Energy Storage Mater., 2016, vol. 5, pp. 139–164.
Hou, W., Guo, X., Shen, X., Amine, K., and Lu, J., Solid electrolytes and interfaces in all-solid-state sodium batteries: progress and perspective, Nano Energy, 2018, vol. 52, pp. 279–291.
Voropaeva, D.Yu., Novikova, S.A., and Yaroslavtsev, A.B., Polymer electrolytes for metal-ion batteries, Russ. Chem. Rev., 2020, vol. 89, no. 10, pp. 1132–1155.
Yaroslavtsev, A.B., Solid electrolytes: main prospects of research and development, Russ. Chem. Rev., 2016, vol. 85, no. 11, pp. 1255–1276.
Zhang, Z., Shao, Y., Lotsch, B., Hu, Y.-S., Li, H., Janek, J., Nazar, L.F., Nan, C.-W., Maier, J., Armand, M., and Chen, L., New horizons for inorganic solid state ion conductors, Energy Environ. Sci., 2018, vol. 11, pp. 1945–1976.
Narimatsu, E., Yamamoto, Y., Takeda, T., Nishimura, T., and Hirosaki, N., High lithium conductivity in Li1 – 2xCaxSi2N3, J. Mater. Res., 2011, vol. 26, pp. 1133–1142.
Li, H., Fan, H., Wang, B., Wang, C., Zhang, M., Chen, G., Jiang, X., Zhao, N., Lu, J., and Zhang, J., Mechanical and electrical properties of lithium stabilized sodium beta alumina solid electrolyte shaping by non-aqueous gelcasting, J. Eur. Ceram. Soc., 2020, vol. 40, pp. 3072–3079.
Xu, L., Li, J., Deng, W., Shuai, H., Li, S., Xu, Z., Li, J., Hou, H., Peng, H., Zou, G., and Ji, X., Garnet solid electrolyte for advanced all-solid-state Li batteries, Adv. Energy Mater., 2021, vol. 11, paper 2000648.
Sastre, J. and Priebe, A., Döbeli, M., Michler, J., Tiwari, A.N., and Romanyuk, Y.E., Lithium garnet Li7La3Zr2O12 electrolyte for all-solid-state batteries: closing the gap between bulk and thin film Li-ion conductivities, Adv. Energy Mater., 2021, vol. 7, paper 2000425.
Kim, A., Woo, S., Kang, M., Park, H., and Kang, B., Research progresses of garnet-type solid electrolytes for developing all-solid-state Li batteries, Front. Chem., 2020.https://doi.org/10.3389/fchem.2020.00468
Catti, M., Sommariva, M., and Ibberson, R.M., Tetragonal superstructure and thermal history of Li0.3La0.567TiO3 (LLTO) solid electrolyte by neutron diffraction, J. Mater. Chem., 2007, vol. 17, pp. 1300–1307.
Zheng, Z., Fang, H., Liu, Z., and Wang, Y., A fundamental stability study for amorphous LiLaTiO3 solid electrolyte, J. Electrochem. Soc., 2015, vol. 162, pp. A244–A248.
Zhang, Y., Zheng, Z., Liu, X., Chi, M., and Wang, Y., Fundamental relationship of microstructure and ionic conductivity of amorphous LLTO as solid electrolyte material, J. Electrochem. Soc., 2019, vol. 166, pp. A515–A520.
Lee, S.J., Bae, J.J., and Son, J.T., Structural and electrical effects of Y-doped Li0.33La0.56 – xYxTiO3 solid electrolytes on all-solid-state lithium ion batteries, J. Kor. Phys. Soc., 2019, vol. 74, pp. 73–77.
Deng, Y., Eames, C., Fleutot, B., David, R., Chotard, J.-N., Suard, E., Masquelier, C., and Islam, M.S., Enhancing the lithium ion conductivity in lithium superionic conductor (LISICON) solid electrolytes through a mixed polyanion effect, ACS Appl. Mater. Interfaces, 2017, vol. 9, pp. 7050–7058.
Hamon, Y., Douard, A., Sabary, F., Marcel, C., Vinatier, P., Pecquenard, B., and Levasseur, A., Influence of sputtering conditions on ionic conductivity of LiPON thin films, Solid State Ionics, 2006, vol. 177, pp. 257–261.
Lacivita, V., Westover, A.S., Kercher, A., Phillip, N.D., Yang, G., Veith, G., Ceder, G., and Dudney, N.J., Resolving the amorphous structure of lithium phosphorus oxynitride (Lipon), J. Am. Chem. Soc., 2018, vol. 140, pp. 11029–11038.
Braga, M.H., Murchison, A.J., Ferreira, J.A., Singh, P., and Goodenough, J.B., Glass-amorphous alkali-ion solid electrolytes and their performance in symmetrical cells, Energy Environ. Sci., 2016, vol. 9, pp. 948–954.
Kato, Y., Hori, S., Saito, T., Suzuki, K., Hirayama, M., Mitsui, A., Yonemura, M., Iba, H., and Kanno, R., High-power all-solid-state batteries using sulfide superionic conductors, Nat. Energy, 2016, vol. 1, pp. 1–7.
Xie, D., Chen, S., Zhang, Z., Ren, J., Yao, L., Wu, L., Yao, X., and Xu, X., High ion conductive Sb2O5-doped B-Li3PS4 with excellent stability against Li for all-solid-state lithium batteries, J. Power Sources, 2018, vol. 389, pp. 140–147.
Huang, W., Cheng, L., Hori, S., Suzuki, K., Yonemura, M., Hirayama, M., and Kanno, R., Ionic conduction mechanism of a lithium superionic argyrodite in the –Al–Si–S–O system, Mater. Adv., 2020, vol. 1, pp. 334–340.
Goodenough, J.B., Hong, H.Y., and Kafalas, J.A., Fast Na+ ion transport in skeleton structures, Mater. Res. Bull., 1976, vol. 11, pp. 203–220.
Das, A., Krishna, P.S.R., Goswami, M., and Krishnan, M., Structural analysis of Al and Si substituted lithium germanium phosphate glass-ceramics using neutron and X-ray diffraction, J. Solid State Chem., 2019, vol. 271, pp. 74–80.
El-Shinawi, H., Regoutz, A., Payne, D.J., Cussen, E.J., and Corr, S.A., NASICON LiM2(PO4)3 electrolyte (M = Zr) and electrode (M = Ti) materials for all solid-state Li-ion batteries with high total conductivity and low interfacial resistance, J. Mater. Chem. A, 2018, vol. 6, pp. 5296–5303.
Rusdi, H., Mohamed, N.S., Subban, R.H.Y., and Rusdi, R., Enhancement of electrical properties of NASICON-type solid electrolytes (LiSn2P3O12) via aluminium substitution, J. Sci. Adv. Mater. Devices, 2020, vol. 5, pp. 368–377.
Pareek, T., Dwivedi, S., Ahmad, S.A., Badole, M., and Kumar, S., Effect of NASICON-type LiSnZr(PO4)3 ceramic filler on the ionic conductivity and electrochemical behavior of PVDF based composite electrolyte, J. Alloys Compd., 2020, vol. 824, paper 153991.
Key, B., Schroeder, D.J., Ingram, B.J., and Vaughey, J.T., Solution-based synthesis and characterization of lithium-ion conducting phosphate ceramics for lithium metal batteries, Chem. Mater., 2012, vol. 24, pp. 287–293.
Svitan’ko, A.I., Novikova, S.A., Stenina, I.A., Skopets, V.A., and Yaroslavtsev, A.B., Microstructure and ion transport in Li1 + xTi2 − xMx(PO4)3 (M = Cr, Fe, Al) NASICON-type materials, Inorg. Mater., 2014, vol. 50, no. 3, pp. 273–279.
Marcinek, M., Syzdek, J., Marczewski, M., Piszcz, M., Niedzicki, L., Kalita, M., Plewa-Marczewska, A., Bitner, A., Wieczorek, P., Trzeciak, T., Kasprzyk, M., Łęzak, P., Zukowska, Z., Zalewska, A., and Wieczorek, W., Electrolytes for Li-ion transport—review, Solid State Ionics, 2015, vol. 276, pp. 107–126.
Yen, P.-Y., Lee, M.-L., Gregory, D.H., and Liu, W.-R., Optimization of sintering process on Li1 + xAlxTi2 – x(PO4)3 solid electrolytes for all-solid-state lithium-ion batteries, Ceram. Int., 2020, vol. 46, pp. 20529–20536.
Bucharsky, E.C., Schell, K.G., Hintennach, A., and Hoffmann, M.J., Preparation and characterization of sol–gel derived high lithium ion conductive NZP-type ceramics Li1 + xAlxTi2 – x(PO4)3, Solid State Ionics, 2015, vol. 274, pp. 77–82.
Zhu, Y., Zhang, Y., and Lu, L., Influence of crystallization temperature on ionic conductivity of lithium aluminum germanium phosphate glass-ceramic, J. Power Sources, 2015, vol. 290, pp. 123–129.
Pershina, S.V., Il’ina, E.A., Druzhinin, K.V., and Farlenkov, A.S., Effect of Li2O–Al2O3–GeO2–P2O5 glass crystallization on stability versus molten lithium, J. Non-Cryst. Solids, 2020, vol. 527, paper 119708.
Stenina, I.A., Kislitsyn, M.N., Pinus, I.Yu., Arkhangel’skii, I.V., Zhuravlev, N.A., and Yaroslavtsev, A.B., Phase transformations and cation mobility in NASICON lithium zirconium double phosphates Li1 ± xZr2 – xMx(PO4)3 (M = Sc, Y, In, Nb, Ta), Russ. J. Inorg. Chem., 2005, vol. 50, no. 6, pp. 906–911.
ACKNOWLEDGMENTS
In this study, we used equipment of the JRC PMR IGIC RAS.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation as part of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurzina, E.A., Stenina, I.A., Dalvi, A. et al. Synthesis and Ionic Conductivity of Lithium Titanium Phosphate-Based Solid Electrolytes. Inorg Mater 57, 1035–1042 (2021). https://doi.org/10.1134/S0020168521100071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521100071