Abstract
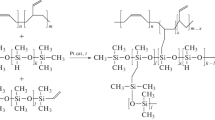
A new membrane for the removal of oxygenates from wastewater by pervaporation has been prepared on the basis of polymethylsiloxane bearing 1-heptene as a substituent on the side chain. The synthesized membrane material has been characterized using Fourier-transform IR spectroscopy, and its sorption properties with respect to C2–C4 alcohols have been examined. It has been found that polyheptylmethylsiloxane (PHepMS) has a greater affinity for the C3 and C4 alcohols to be separated than its closest analogue known from the literature (polyoctylmethylsiloxane (POMS)), which makes the PHepMS membrane promising for the pervaporative separation of aqueous solutions of these alcohols. The pervaporation properties of PHepMS have been studied for the first time, and its separation characteristics have been compared with those of the commercial highly permeable membrane polymer polydimethylsiloxane (PDMS) and POMS in relation to the problem of recovery of n-butanol, n-propanol, and ethanol from dilute aqueous solutions by vacuum pervaporation. It has been shown that PDMS has the highest separation efficiency for n-propanol–water mixture and PHepMS is the most promising membrane material for the pervaporative separation of water–butanol mixtures. Having a butanol flux comparable to that through PDMS, the PHepMS membrane demonstrates a record-breaking value of butanol/water separation factor of 97.




Similar content being viewed by others
REFERENCES
R. A. Deeb, K. H. Chu, T. Shih, et al., Environ. Eng. Sci. 20, 433 (2003).
L. C. Davis and L. E. Erickson, Environ. Prog. 23, 243 (2004).
M. M. Zein, P. X. Pinto, S. Garcia-Blanco, et al., Biodegradation 17, 57 (2006).
G. V. Porutskii, Biochemical Treatment of Wastewater from Organic Productions (Khimiya, Moscow, 1975) [in Russian].
M. J. Tijmensen, A. P. Faaij, C. N. Hamelinck, and M. R. Van Hardeveld, Biomass Bioenergy 23, 129 (2002).
Mémento Technique de l’Eau, 10th Ed. (Degrémont, Paris, 2005), Vol. 1.
F. Fayolle, J. P. Vandecasteele, and F. Monot, Appl. Microbiol. Biotechnol. 56, 339 (2001).
H. Kölbel and M. Ralek, Catal. Rev. 21, 225 (1980).
A. de Klerk, Fischer–Tropsch Refining (Weinheim, Wiley–VCH, 2012).
A. Yu. Krylova, Yu. G. Kryazhev, M. V. Kulikova, et al., Solid Fuel Chem. 45, 32 (2011).
T. C. Ezeji, N. Qureshi, and H. P. Blaschek, Chem. Rec. 4, 305 (2004).
G. Liu, W. Wei, and W. Jin, ACS Sustain. Chem. Eng. 2, 546 (2013).
L. M. Vane, J. Chem. Technol. Biotechnol. 80, 603 (2005).
T. Ikegami, H. Yanagishita, D. Kitamoto, et al., Biotechnol. Tech. 11, 921 (1997).
A. Rozicka, J. Niemisto, R. L. Keiski, and W. Kujawski, J. Membr. Sci. 453, 108 (2014).
A. Rom and A. Friedl, Sep. Purif. Technol. 170, 40 (2016).
I. L. Borisov, N. V. Ushakov, V. V. Volkov, and E. Sh. Finkel’shtein, Pet. Chem. 56, 800 (2016).
J. Schultz and K.-V. Peinemann, J. Membr. Sci. 110, 37 (1996).
M. Žák, M. Klepic, L. Č. Štastná, et al., Sep. Purif. Technol. 151, 108 (2015).
I. L. Borisov, A. O. Malakhov, V. S. Khotimsky, et al., J. Membr. Sci. 466, 322 (2014).
W. Van Hecke and H. de Wever, J. Membr. Sci. 540, 321 (2017).
J. Börjesson, H. O. E. Karlsson, and G. Trägårdh, J. Membr. Sci. 119, 229 (1996).
S. A. Stern, V. M. Shah, and B. J. Hardy, J. Polym. Sci., Part B: Polym. Phys. 25, 1263 (1987).
E. A. Grushevenko, I. L. Borisov, D. S. Bakhtin, et al., Pet. Chem. 57, 334 (2017).
S. Darvishmanesh, J. Degréve, and B. Van der Bruggen, Chem. Eng. Sci. 64, 3914 (2009).
R. W. Baker, J. G. Wijmans, and Y. Huang, J. Membr. Sci. 348, 346 (2010).
A. Kujawska, K. Knozowska, J. Kujawa, and W. Kujawski, Sep. Purif. Technol. 159, 68 (2016).
I. L. Borisov, G. S. Golubev, V. P. Vasilevsky, et al., J. Membr. Sci. 523, 291 (2017).
S. Darvishmanesh, J. Degreve, and B. Van der Bruggen, Phys. Chem. Chem. Phys. 12, 13333 (2010).
D. W. van Krevelen and K. Nijenhuis, Properties of Polymers, 4th Ed. (Elsevier Science, Amsterdam, 2009).
E. S. Tarleton, J. P. Robinson, and J. J. W. Na, J. Membr. Sci. 261, 129 (2005).
C. M. Hansen, Hansen Solubility Parameters: A User’s Handbook, 2nd Ed. (CRC, Boca Raton, 2007).
M. Bennett, B. J. Brisdon, R. England, and R. W. Field, J. Membr. Sci. 137, 63 (1997).
B. Van der Bruggen and P. Luis, Progress in Filtration and Separation, Ed. by E. S. Tarleton (Academic, London, 2015), p. 101.
D. J. O’Brien, L. H. Roth, and A. J. McAloon, J. Membr. Sci. 166, 105 (2000).
L. M. Vane, Sep. Sci. Technol. 48, 429 (2013).
ACKNOWLEDGMENTS
This work was supported by the Russian Science Foundation, project no. 17-79-20296. The authors are grateful to G.N. Bondarenko for making FTIR measurements and to the Center for Collective Use at the Topchiev Institute for the equipment provided.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Grushevenko, E.A., Podtynnikov, I.A., Golubev, G.S. et al. Polyheptylmethylsiloxane—A Novel Material for Removal of Oxygenates from Water by Pervaporation. Pet. Chem. 58, 941–948 (2018). https://doi.org/10.1134/S0965544118110026
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544118110026