Abstract
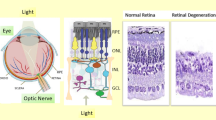
Both vertebrates and invertebrates respond to light by utilising a wide-ranging array of photosensory systems, with diverse photoreceptor organs expressing a characteristic photopigment, itself consisting of an opsin apoprotein linked to a light-sensitive retinoid chromophore based on vitamin A. In the eye, the pigments expressed in both cone and rod photoreceptors have been studied in great depth and mediate contrast perception, measurement of the spectral composition of environmental light, and thus classical image forming vision. By contrast, the molecular basis for non-visual and extraocular photoreception is far less understood; however, two photopigment genes have become the focus of much study, the vertebrate ancient (va) opsin and melanopsin (opn4). In this review, we discuss the history of discovery for each gene, as well as focusing on the evolution, expression profile, functional role and broader physiological significance of each photopigment. Recently, it has been suggested independently by Arendt et al. and Lamb that an ancestral opsin bifurcated in early metazoans and evolved into two quite different photopigments, one expressed in rhabdomeric photoreceptors and the other in ciliary photoreceptors. This interpretation of the evolution of the metazoan eye has provided a powerful framework for understanding photobiological organization. Their proposal, however, does not encompass all current experimental observations that would be consistent with what we term a central “Evolution of Photosensory Opsins with Common Heredity (EPOCH)” hypothesis to explain the complexity of animal photosensory systems. Clearly, many opsin genes (e.g. va opsin) simply do not fit neatly within this scheme. Thus, the review concludes with a discussion of these anomalies and their context regarding the phylogeny of photoreceptor and photopigment development.
Similar content being viewed by others
References
D. Arendt, The evolution of cell types in animals: emerging principles from molecular studies, Nat. Rev. Genet., 2008, 9, 868–882.
B. G. Soni, A. R. Philp, B. E. Knox, R. G. Foster, Novel retinal photoreceptors, Nature, 1998, 394, 27–28.
D. M. Berson, F. A. Dunn, M. Takao, Phototransduction by retinal ganglion cells that set the circadian clock, Science, 2002, 295, 1070–1073.
S. Sekaran, R. G. Foster, R. J. Lucas, M. W. Hankins, Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons, Curr. Biol., 2003, 13, 1290–1298.
I. Provencio, G. Jiang, W. J. DeGrip, W. P. Hayes, M. D. Rollag, Melanopsin: An opsin in melanophores, brain and eye, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 340–345.
S. N. Peirson, P. H. Bovee-Geurts, D. Lupi, G. Jeffery, W. J. DeGrip, R. G. Foster, Expression of the candidate circadian photopigment melanopsin (Opn4) in the mouse retinal pigment epithelium, Mol. Brain Res., 2004, 123, 132–135.
L. Vollrath, in The Pineal Organ, ed. L. Vollrath and A. Oksche, Springer International Publishing, Berlin, Heidelberg, New York, 1981.
S. Halford, S. S. Pires, M. Turton, L. Zheng, I. Gonzalez-Menendez, W. L. Davies, S. N. Peirson, J. M. Garcia-Fernandez, M. W. Hankins, R. G. Foster, VA opsin-based photoreceptors in the hypothalamus of birds, Curr. Biol., 2009, 19, 1396–1402.
D. Whitmore, N. S. Foulkes, P. Sassone-Corsi, Light acts directly on organs and cells in culture to set the vertebrate circadian clock, Nature, 2000, 404, 87–91.
M. D. Rollag, I. Provencio, D. Sugden, C. B. Green, Cultured amphibian melanophores: a model system to study melanopsin photobiology, Methods Enzymol., 2000, 316, 291–309.
J. Shand, J. N. Lythgoe, The isolated iridescent cornea of the sand goby is photoresponsive, Photochem. Photobiol., 1990, 51, 737–739.
M. S. Freedman, R. J. Lucas, B. Soni, M. von Schantz, M. Munoz, Z. David-Gray, R. G. Foster, Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors, Science, 1999, 284, 502–504.
D. Lupi, H. Oster, S. Thompson, R. G. Foster, The acute light-induction of sleep is mediated by OPN4-based photoreception, Nat. Neurosci., 2008, 11, 1068–1073.
A. Engbretson, Neurobiology of the lacertilian parietal eye system, Ethol. Ecol. Evol., 1992, 4, 89–107.
J. T. Bagnara, M. E. Hadley, Endocrinology of the amphibian pineal, Am. Zool., 1970, 10, 201–216.
G. Tosini, M. Menaker, The pineal complex and melatonin affect the expression of the daily rhythm of behavioural thermoregulation in the green iguana, J. Comp. Physiol., A, 1996, 179, 135–142.
R. J. Lucas, R. H. Douglas, R. G. Foster, Characterization of an ocular photopigment capable of driving pupillary constriction in mice, Nat. Neurosci., 2001, 4, 621–626.
R. G. Foster, M. W. Hankins, Non-rod, non-cone photoreception in the vertebrates, Prog. Retinal Eye Res., 2002, 21, 507–527.
I. Provencio, H. M. Cooper, R. G. Foster, Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment, J. Comp. Neurol., 1998, 395, 417–439.
T. S. Kemp, The origin and early radiation of the therapsid mammallike reptiles: A palaeobiological hypothesis, J. Evol. Biol., 2006, 19, 1231–1247.
S. G. Lucas, Z. Lou, Adelobasileus from the upper Triassic of west Texas: the oldest mammal, J. Vertebr. Paleontol., 1993, 13, 309–334.
J. Z. Young, in The Life of Vertebrates, ed. M. Nixon, Oxford University Press, Oxford, 3rd edn, 1981.
R. G. Foster, M. Menaker, in Light and biological rhythms in man, ed. L. Wetterberg, Pergamon, 1993, pp. 73–91.
J. Bellingham, R. G. Foster, Opsins and mammalian photoentrainment, Cell Tissue Res., 2002, 309, 57–71.
A. Terakita, The opsins, GenomeBiology, 2005, 6, 213.
B. Nickle, P. R. Robinson, The opsins of the vertebrate retina: insights from structural, biochemical, and evolutionary studies, Cell. Mol. Life Sci., 2007, 64, 2917–2932.
M. W. Hankins, S. N. Peirson, R. G. Foster, Melanopsin: an exciting photopigment, Trends Neurosci., 2008, 31, 27–36.
T. D. Lamb, Evolution of vertebrate retinal photoreception, Philos. Trans. R. Soc. London, Ser. B, 2009, 364, 2911–2924.
S. N. Peirson, S. Halford, R. G. Foster, The evolution of irradiance detection: melanopsin and non-visual opsins, Philos. Trans. R. Soc. London, Ser. B, 2009, 364, 2849–2865.
K. D. Ridge, K. Palczewski, Visual rhodopsin sees the light: structure and mechanism of G protein signaling, J. Biol. Chem., 2007, 282, 9297–9301.
B. G. Soni, R. G. Foster, A novel and ancient vertebrate opsin, FEBS Lett., 1997, 406, 279–283.
T. Okano, T. Yoshizawa, Y. Fukada, Pinopsin is a chicken pineal photoreceptive molecule, Nature, 1994, 372, 94–97.
M. Max, P. J. McKinnon, K. J. Seidenman, R. K. Barrett, M. L. Applebury, J. S. Takahashi, R. F. Margolskee, Pineal opsin: a nonvisual opsin expressed in chick pineal, Science, 1995, 267, 1502–1506.
P. Moutsaki, J. Bellingham, B. G. Soni, Z. K. David-Gray, R. G. Foster, Sequence, genomic structure, and tissue expression of carp (Cyprinus carpio L.) vertebrate ancient (VA) opsin, FEBS Lett., 2000, 473, 316–322.
D. Kojima, H. Mano, Y. Fukada, Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells, J. Neurosci., 2000, 20, 2845–2851.
D. Kojima, M. Torii, Y. Fukada, J. E. Dowling, Differential expression of duplicated VAL-opsin genes in developing zebrafish, J. Neurochem., 2008, 104, 1364–1371.
T. Minamoto, I. Shimizu, A novel isoform of vertebrate ancient opsin in a smelt fish Plecoglossus altivelis, Biochem. Biophys. Res. Commun., 2002, 290, 280–286.
S. Yokoyama, H. Zhang, Cloning and characterization of the pineal gland-specific opsin gene of marine lamprey (Petromyzon marinus), Gene, 1997, 202, 89–93.
A. R. Philp, J. M. Garcia-Fernandez, B. G. Soni, R. J. Lucas, J. Bellingham, R. G. Foster, Vertebrate ancient (VA) opsin and extraretinal photoreception in the Atlantic salmon (Salmo salar), J. Exp. Biol., 2000, 203, 1925–1936.
A. Jenkins, M. Munoz, E. E. Tarttelin, J. Bellingham, R. G. Foster, M. W. Hankins, VA opsin, melanopsin, and an inherent light response within retinal interneurons, Curr. Biol., 2003, 13, 1269–1278.
N. Cheng, T. Tsunenari, K. W. Yau, Intrinsic light response of retinal horizontal cells of teleosts, Nature, 2009, 460, 899–903.
J. Forsell, P. Ekstrom, I. N. Flamarique, B. Holmqvist, Expression of pineal ultraviolet- and green-like opsins in the pineal organ and retina of teleosts, J. Exp. Biol., 2001, 204, 2517–2525.
P. Ekstrom, H. Meissl, Evolution of photosensory pineal organs in new light: the fate of neuroendocrine photoreceptors, Philos. Trans. R. Soc. London, Ser. B, 2003, 358, 1679–1700.
Z. Melyan, E. E. Tarttelin, J. Bellingham, R. J. Lucas, M. W. Hankins, Addition of human melanopsin renders mammalian cells photoresponsive, Nature, 2005, 433, 741–745.
J. Benoit, Stimulation par la lumiere artificielle du developpement testiculaire chez des canards aveugles par section du nerf optique, C. R. Seances. Soc. Biol. Fil., 1935, 120, 133–136.
J. Benoit, Le role des yeux dans l’action stimulante de la lumiere sur le developpement testiculaire chez le canard, C. R. Seances. Soc. Biol. Fil., 1935, 118, 669–671.
T. Yoshimura, S. Yasuo, M. Watanabe, M. Iigo, T. Yamamura, K. Hirunagi, S. Ebihara, Light-induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds, Nature, 2003, 426, 178–181.
N. Nakao, H. Ono, T. Yamamura, T. Anraku, T. Takagi, K. Higashi, S. Yasuo, Y. Katou, S. Kageyama, Y. Uno, T. Kasukawa, M. Iigo, P. J. Sharp, A. Iwasawa, Y. Suzuki, S. Sugano, T. Niimi, M. Mizutani, T. Namikawa, S. Ebihara, H. R. Ueda, T. Yoshimura, Thyrotrophin in the pars tuberalis triggers photoperiodic response, Nature, 2008, 452, 317–322.
R. G. Foster, B. K. Follett, J. N. Lythgoe, Rhodopsin-like sensitivity of extra-retinal photoreceptors mediating the photoperiodic response in quail, Nature, 1985, 313, 50–52.
R. G. Foster, H. G. Korf, J. J. Schalken, Immunocytochemical markers revealing retinal and pineal but not hypothalamic photoreceptor systems in the Japanese quail, Cell Tissue Res., 1987, 248, 161–167.
R. M. Das, N. J. Van Hateren, G. R. Howell, E. R. Farrell, F. K. Bangs, V. C. Porteous, E. M. Manning, M. J. McGrew, K. Ohyama, M. A. Sacco, P. A. Halley, H. M. Sang, K. G. Storey, M. Placzek, C. Tickle, V. K. Nair, S. A. Wilson, A robust system for RNA interference in the chicken using a modified microRNA operon, Dev. Biol., 2006, 294, 554–563.
M. O. Woodburne, T. H. Rich, M. S. Springer, The evolution of tribospheny and the antiquity of mammalian clades, Mol. Phylogenet. Evol., 2003, 28, 360–385.
I. Provencio, I. R. Rodriguez, G. Jiang, W. P. Hayes, E. F. Moreira, M. D. Rollag, A novel human opsin in the inner retina, J. Neurosci., 2000, 20, 600–605.
I. Provencio, M. D. Rollag, A. M. Castrucci, Photoreceptive net in the mammalian retina, Nature, 2002, 415, 493.
M. Koyanagi, K. Kubokawa, H. Tsukamoto, Y. Shichida, A. Terakita, Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells, Curr. Biol., 2005, 15, 1065–1069.
B. P. Grone, Z. Sheng, C. C. Chen, R. D. Fernald, Localization and diurnal expression of melanopsin, vertebrate ancient opsin, and pituitary adenylate cyclase-activating peptide mRNA in a teleost retina, J. Biol. Rhythms, 2007, 22, 558–561.
O. Drivenes, A. M. Soviknes, L. O. Ebbesson, A. Fjose, H. C. Seo, J. V. Helvik, Isolation and characterization of two teleost melanopsin genes and their differential expression within the inner retina and brain, J. Comp. Neurol., 2003, 456, 84–93.
J. Bellingham, D. Whitmore, A. R. Philp, D. J. Wells, R. G. Foster, Zebrafish melanopsin: isolation, tissue localisation and phylogenetic position, Mol. Brain Res., 2002, 107, 128–136.
E. Frigato, D. Vallone, C. Bertolucci, N. S. Foulkes, Isolation and characterization of melanopsin and pinopsin expression within photoreceptive sites of reptiles, Naturwissenschaften, 2006, 93, 379–385.
S. S. Chaurasia, M. D. Rollag, G. Jiang, W. P. Hayes, R. Haque, A. Natesan, M. Zatz, G. Tosini, C. Liu, H. W. Korf, P. M. Iuvone, I. Provencio, Molecular cloning, localization and circadian expression of chicken melanopsin (Opn4): differential regulation of expression in pineal and retinal cell types, J. Neurochem., 2005, 92, 158–170.
J. Bellingham, S. S. Chaurasia, Z. Melyan, C. Liu, M. A. Cameron, E. E. Tarttelin, P. M. Iuvone, M. W. Hankins, G. Tosini, R. J. Lucas, Evolution of melanopsin photoreceptors: discovery and characterization of a new melanopsin in nonmammalian vertebrates, PLoS Biol., 2006, 4, e254.
S. S. Pires, J. Shand, J. Bellingham, C. A. Arrese, M. Turton, S. N. Peirson, R. G. Foster, S. Halford, Isolation and characterization of melanopsin (Opn4) from the Australian marsupial Sminthopsis crassicaudata (fat-tailed dunnart), Proc. R. Soc. London, Ser. B, 2007, 274, 2791–2799.
S. S. Pires, S. Hughes, M. Turton, Z. Melyan, S. N. Peirson, L. Zheng, M. Kosmaoglou, J. Bellingham, M. E. Cheetham, R. J. Lucas, R. G. Foster, M. W. Hankins, S. Halford, Differential expression of two distinct functional isoforms of melanopsin (Opn4) in the mammalian retina, J. Neurosci., 2009, 29, 12332–12342.
S. Hattar, H. W. Liao, M. Takao, D. M. Berson, K. W. Yau, Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity, Science, 2002, 295, 1065–1070.
R. Hermann, L. Poppe, S. Pilbak, C. Boden, J. Maurer, S. Weber, A. Lerchl, Predicted 3D-structure of melanopsin, the non-rod, non-cone photopigment of the mammalian circadian clock, from Djungarian hamsters (Phodopus sungorus), Neurosci. Lett., 2005, 376, 76–80.
M. Semo, M. Munoz, R. G. Foster, G. Jeffery, Melanopsin (Opn4) positive cells in the cat retina are randomly distributed across the ganglion cell layer, Visual Neurosci., 2005, 22, 111–116.
R. G. Foster and L. Kreitzman, in Rhythms of Life: The biological clocks that control the daily lives of every living thing, Profile Books, London, 2004.
R. G. Foster, I. Provencio, D. Hudson, S. Fiske, W. DeGrip, M. Menaker, Circadian photoreception in the retinally degenerate mouse (rd/rd), J. Comp. Physiol., A, 1991, 169, 39–50.
I. Provencio, S. Wong, A. B. Lederman, S. M. Argamaso, R. G. Foster, Visual and circadian responses to light in aged retinally degenerate mice, Vision Res., 1994, 34, 1799–1806.
C. A. Czeisler, T. L. Shanahan, E. B. Klerman, H. Martens, D. J. Brotman, J. S. Emens, T. Klein, J. F. Rizzo, 3rd, Suppression of melatonin secretion in some blind patients by exposure to bright light, N. Engl. J. Med., 1995, 332, 6–11.
R. J. Lucas, M. S. Freedman, M. Munoz, J. M. Garcia-Fernandez, R. G. Foster, Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors, Science, 1999, 284, 505–507.
S. Hattar, R. J. Lucas, N. Mrosovsky, S. Thompson, R. H. Douglas, M. W. Hankins, J. Lem, M. Biel, F. Hofmann, R. G. Foster, K. W. Yau, Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice, Nature, 2003, 424, 75–81.
D. M. Dacey, H. W. Liao, B. B. Peterson, F. R. Robinson, V. C. Smith, J. Pokorny, K. W. Yau, P. D. Gamlin, Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN, Nature, 2005, 433, 749–754.
M. W. Hankins, R. J. Lucas, The primary visual pathway in humans is regulated according to long-term light exposure through the action of a non-classical photopigment, Curr. Biol., 2002, 12, 191–198.
R. J. Lucas, S. Hattar, M. Takao, D. M. Berson, R. G. Foster, K. W. Yau, Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice, Science, 2003, 299, 245–247.
S. Panda, T. K. Sato, A. M. Castrucci, M. D. Rollag, W. J. DeGrip, J. B. Hogenesch, I. Provencio, S. A. Kay, Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting, Science, 2002, 298, 2213–2216.
S. Panda, I. Provencio, D. C. Tu, S. S. Pires, M. D. Rollag, A. M. Castrucci, M. T. Pletcher, T. K. Sato, T. Wiltshire, M. Andahazy, S. A. Kay, R. N. Van, Gelder, J. B. Hogenesch, Melanopsin is required for non-image-forming photic responses in blind mice, Science, 2003, 301, 525–527.
N. F. Ruby, T. J. Brennan, X. Xie, V. Cao, P. Franken, H. C. Heller, H. F. O’Hara, Role of melanopsin in circadian responses to light, Science, 2002, 298, 2211–2213.
X. Qiu, T. Kumbalasiri, S. M. Carlson, K. Y. Wong, V. Krishna, I. Provencio, D. M. Berson, Induction of photosensitivity by heterologous expression of melanopsin, Nature, 2005, 433, 745–749.
M. Torii, D. Kojima, T. Okano, A. Nakamura, A. Terakita, Y. Shichida, A. Wada, Y. Fukada, Two isoforms of chicken melanopsins show blue light sensitivity, FEBS Lett., 2007, 581, 5327–5331.
S. Panda, S. K. Nayak, B. Campo, J. R. Walker, J. B. Hogenesch, T. Jegla, Illumination of the melanopsin signaling pathway, Science, 2005, 307, 600–604.
A. Terakita, H. Tsukamoto, M. Koyanagi, M. Sugahara, T. Yamashita, Y. Shichida, Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin, J. Neurochem., 2008, 105, 883–890.
S. Sekaran, G. S. Lall, K. l. Ralphs, A. J. Wolstenholme, R. J. Lucas, R. G. Foster, M. W. Hankins, 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo, J. Neurosci., 2007, 27, 3981–3986.
S. N. Peirson, H. Oster, S. L. Jones, M. Leitges, M. W. Hankins, R. G. Foster, Microarray analysis and functional genomics identify novel components of melanopsin signaling, Curr. Biol., 2007, 17, 1363–1372.
I. Provencio, R. G. Foster, Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics, Brain Res., 1995, 694, 183–190.
Z. K. David-Gray, J. W. Janssen, W. J. DeGrip, E. Nevo, R. G. Foster, Light detection in a ‘blind’ mammal, Nat. Neurosci., 1998, 1, 655–656.
P. M. Smallwood, B. P. Olveczky, G. L. Williams, G. H. Jacobs, B. E. Reese, M. Meister, J. Nathans, Genetically engineered mice with an additional class of cone photoreceptors: Implications for the evolution of color vision, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 11706–11711.
G. S. Lall, V. L. Revell, H. Momiji, J. A. Enezi, C. M. Altimus, A. D. Guler, C. Aguilar, M. A. Cameron, S. Allender, M. W. Hankins, R. J. Lucas, Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance, Neuron, 2010, 66, 417–428.
S. Sekaran, D. Lupi, S. L. Jones, C. J. Sheely, S. Hattar, K. W. Yau, R. J. Lucas, R. G. Foster, M. W. Hankins, Melanopsin-dependent photoreception provides earliest light detection in the mammalian retina, Curr. Biol., 2005, 15, 1099–1107.
J. Nathans, D. S. Hogness, Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin, Cell, 1983, 34, 807–814.
I. M. Pepe, Rhodopsin and phototransduction, J. Photochem. Photobiol., B, 1999, 48, 1–10.
S. S. Karnik, T. P. Sakmar, H. B. Chen, H. G. Khorana, Cysteine residues 110 and 187 are essential for the formation of correct structure in bovine rhodopsin, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 8459–8463.
R. R. Franke, T. P. Sakmar, R. M. Graham, H. G. Khorana, Structure and function in rhodopsin. Studies of the interaction between the rhodopsin cytoplasmic domain and transducin, J. Biol. Chem., 1992, 267, 14767–14774.
P. A. Hargrave, The amino-terminal tryptic peptide of bovine rhodopsin. A glycoprotein containing two sites of oligosaccharide attachment, Biochim. Biophys. Acta, Protein Struct., 1977, 492, 83–94.
Y. A. Ovchinnikov, N. G. Abdulaev, A. S. Bogachuk, Two adjacent cysteine residues in the C-terminal fragment of bovine rhodopsin are palmitoylated, FEBS Lett., 1988, 230, 1–5.
O. Fritze, S. Filipek, V. Kuksa, K. Palczewski, K. P. Hofmann, O. P. Ernst, Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 2290–2295.
K. Palczewski, T. Kumasaka, T. Hori, C. A. Behnke, H. Motoshima, B. A. Fox, I. L. Trong, D. C. Teller, T. Okada, R. E. Stenkamp, M. Yamamoto, M. Miyano, Crystal structure of rhodopsin: A G protein-coupled receptor, Science, 2000, 289, 739–745.
A. Terakita, M. Koyanagi, H. Tsukamoto, T. Yamashita, T. Miyata, Y. Shichida, Counterion displacement in the molecular evolution of the rhodopsin family, Nat. Struct. Mol. Biol., 2004, 11, 284–289.
Z. Wang, A. B. Asenjo, D. D. Oprian, Identification of the Cl(−)-binding site in the human red and green color vision pigments, Biochemistry, 1993, 32, 2125–2130.
S. Yokoyama, H. Yang, W. T. Starmer, Molecular basis of spectral tuning in the red- and green-sensitive (M/LWS) pigments in vertebrates, Genetics, 2008, 179, 2037–2043.
H. Sun, J. P. Macke, J. Nathans, Mechanisms of spectral tuning in the mouse green cone pigment, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 8860–8865.
D. Arendt, H. Hausen, G. Purschke, The ‘division of labour’ model of eye evolution, Philos. Trans. R. Soc. London, Ser. B, 2009, 364, 2809–2817.
Z. Kozmik, J. Ruzickova, K. Jonasova, Y. Matsumoto, P. Vopalensky, I. Kozmikova, H. Strnad, S. Kawamura, J. Piatigorsky, V. Paces, C. Vlcek, Assembly of the cnidarian camera-type eye from vertebrate-like components, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8989–8993.
M. Koyanagi, K. Takano, H. Tsukamoto, K. Ohtsu, F. Tokunaga, A. Terakita, Jellyfish vision starts with cAMP signalling mediated by opsin-Gs cascade, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 15576–15580.
S. R. Das, N. Bhardwaj, H. Kjeldbye, P. Gouras, Muller cells of chicken retina synthesize 11-cis-retinol, Biochem. J., 1992, 285, 907–913.
B. X. Wu, G. Moiseyev, Y. Chen, B. Rohrer, R. K. Crouch, J. X. Ma, Identification of RDH10, an All-trans Retinol Dehydrogenase, in Retinal Muller Cells, Invest. Ophthalmol. Visual Sci., 2004, 45, 3857–3862.
R. G. Foster, J. J. Schalken, A. M. Timmers, W. J. DeGrip, A comparison of some photoreceptor characteristics in the pineal and retina: I. The, Japanese quail (Coturnix coturnix), J. Comp. Physiol., A, 1989, 165, 553–563.
T. G. Kusakabe, N. Takimoto, M. Jin, M. Tsuda, Evolution and the origin of the visual retinoid cycle in vertebrates, Philos. Trans. R. Soc. London, Ser. B, 2009, 364, 2897–2910.
E. A. Griffin Jr, D. Staknis, C. J. Weitz, Light-independent role of CRY1 and CRY2 in the mammalian circadian clock, Science, 1999, 286, 768–771.
E. van der Schalie, C. B. Green, Cryptochromes, Curr. Biol., 2005, 15, R785.
D. G. Higgins, J. D. Thompson, T. J. Gibson, Using CLUSTAL for multiple sequence alignments, Methods Enzymol., 1996, 266, 383–402.
M. Nei and S. Kumar, in Molecular Evolution and Phylogenetics, Oxford University Press, Oxford, UK, 2000.
K. Tamura, J. Dudley, M. Nei, S. Kumar, MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0, Mol. Biol. Evol., 2007, 24, 1596–1599.
Author information
Authors and Affiliations
Additional information
This article is published as part of a themed issue on photosensitive visual pigments: opsins and retinoids.
Rights and permissions
About this article
Cite this article
Davies, W.L., Hankins, M.W. & Foster, R.G. Vertebrate ancient opsin and melanopsin: divergent irradiance detectors. Photochem Photobiol Sci 9, 1444–1457 (2010). https://doi.org/10.1039/c0pp00203h
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c0pp00203h