Abstract
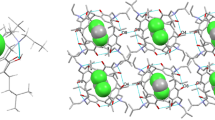
The crystal structure of the molecular complex of C-undecylcalix[4]resorcinarene with dioxane has been determined by X-ray analysis. The asymmetric unit contains one host and four guest molecules. The calix[4]resorcinarene moiety adopts a bowl conformation with C4v symmetry. Four undecyl chains are axially oriented. Calix molecules are packed in a bowl-to-bowl fashion with alternating hydrophilic and hydrophobic layers. One of the ‘hydrophilic’ dioxane molecules is located at the rim of the calix moiety and is hydrogen bonded to the other one. There is no interaction to attract, or direct the dioxane molecule into the interior of the cavity. There is an exo complex formed. The dioxane molecules – located in the hydrophobic part – are highly disordered.
Similar content being viewed by others
References
Y. Aoyama, Y. Tanaka, H. Toi, and H. Ogoshi: J. Am. Chem. Soc. 110, 634 (1988); Y. Aoyama, T. Uzawa, K. Saita, Y. Tanaka, H. Toi, H. Ogoshi, and Y. Okamoto: Tetrahedron Lett. 29, 5271 (1988); Y. Aoyama, Y. Tanaka, and S. Sugahara: J. Am. Chem. Soc. 111, 5397 (1989); K. Kurihara, K. Ohto, Y. Tanaka, Y. Aoyama, and T. Kunitake: J. Am. Chem. Soc. 113, 444 (1991); U. Schneider and H.-J. Schneider: Chem. Ber. 127, 2455 (1994); D. A. Leigh, P. Linnane, R. G. Pritchard, and G. Jackson: J. Chem. Soc., Chem. Commun. 389, (1994); H.-J. Schneider and U. Schneider: J. Incl. Phenom. & Mol. Recogn. Chem. 19, 67 (1994).
Y. Koide, H. Sato, H. Shosenji, and K. Yamada: Bull. Chem. Soc. Jap. 69, 315 (1996); T. Jin, M. Kinjo, T. Koyama, Y. Kobayashi, and H. Hirata: Langmuir 12, 2684 (1996).
Y. Tanaka, Y. Kobuke, and M. Sokabe: Angew. Chem. Int. Ed. Engl. 34, 693 (1995).
W. C. Moreira, P. J. Dutton, and R. Aroca: Langmuir 10, 4148 (1994); K. Kurihara, K. Ohto, Y. Tanaka, Y. Aoyama, and T. Kunitake: J. Am. Chem. Soc. 113, 444 (1991); F. Davis and C. J. M. Stirling: Langmuir 12, 5365 (1996); W. C. Moreira, P. J. Dutton, and R. Aroca: Langmuir 11, 3137 (1995).
E. U. T. Van Velzen, J. F. J. Engbersen, P. J. de Lange, J. W. G. Mahy, and D. N. Reinhoudt: J. Am. Chem. Soc. 117, 6853 (1995); E. U. T. van Velzen, J. F. J. Engbersen, and D. N. Reinhoudt: Synthesis 989 (1995); B.-H. Huisman, R. P. H. Kooyman, F. C. J. M. van Veggel, and D. N. Reinhoudt: Adv. Mater. 7, 561 (1996); H. Schoenherr, G. J. Vancso, B.-H. Huisman, F. C. J. M. van Veggel, and D. N. Reinhoudt: Langmuir 13, 1567 (1997); H. Adams, F. Davis, and C. J. M. Stirling: J. Chem. Soc., Chem. Commun. 1527 (1994).
P. Timmerman, W. Verboom, and D. N. Reinhoudt: Tetrahedron 52, 2663 (1996).
F. H. Allen, J. E. Davies, J. J. Galloy, O. Johnson, O. Kennard, C. F. Macrae, E. M. Mitchell, G. F. Mitchell, J. M. Smith, and D. G. Watson: J. Chem. Inf. Comput. Sci. 31, 187 (1991).
L. M. Tunstad, J. A. Tucker, E. Dalcanale, J. Weiser, J. A. Bryant, J. C. Sherman, R. C. Helgeson, C. B. Knobler, and D. J. Cram: J. Org. Chem. 54, 1305 (1989). G. Zahn, J. Sieler, K. Müller, L. Henning, and G. Mann: Z. Kristallogr. 209, 468 (1994).
H. Adams, F. Davis, and C. J. M. Stirling: J. Chem. Soc., Chem. Commun. 2527 (1994). K. Murayama and K. Aoki: J. Chem. Soc., Chem. Commun. 119 (1997).
T. Lippmann, H. Wilde, M. Pink, A. Schafer, M. Hesse, and G. Mann: Angew. Chem., Int. Ed. 32, 1195 (1993).
D. A. Leigh, P. Linnane, R. G. Pritchard, and G. Jackson: J. Chem. Soc., Chem. Commun. 389 (1994).
A. Shivanyuk, E. F. Paulus, V. Bohmer, and W. Vogt: Angew. Chem., Int. Ed. 36, 1301 (1997).
Y. Aoyama, Y. Tanaka, H. Toi, and H. Ogoshi: J. Am. Chem. Soc. 110, 634 (1988).
Kuma KM-4 Software, User's Guide, Version 5.0. Kuma Diffraction, Wrocław, Poland 1991.
G. M. Sheldrick: Acta Crystallogr. A46, 467 (1990).
G. M. Sheldrick (1997): SHELXL97._Program for the Refinement of Crystal Structures. University of Göttingen, Germany.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borowia, T., Mączyński, M., Pietraszkiewicz, M. et al. Structural Study of C-undecylcalix[4]resorcinarene Solvate with Dioxane. Journal of Inclusion Phenomena 35, 131–138 (1999). https://doi.org/10.1023/A:1008115002434
Issue Date:
DOI: https://doi.org/10.1023/A:1008115002434