Abstract
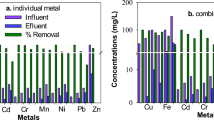
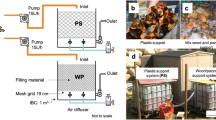
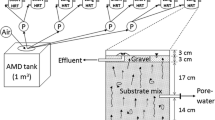
Several biotreatmemt techniques for sulfate conversion by the sulfate reducing bacteria (SRB) have been proposed in the past, however few of them have been practically applied to treat sulfate containing acid mine drainage (AMD). This research deals with development of an innovative polypropylene hollow fiber membrane bioreactor system for the treatment of acid mine water from the Berkeley Pit, Butte, MT, using hydrogen consuming SRB biofilms. The advantages of using the membrane bioreactor over the conventional tall liquid phase sparged gas bioreactor systems are: large microporous membrane surface to the liquid phase; formation of hydrogen sulfide outside the membrane, preventing the mixing with the pressurized hydrogen gas inside the membrane; no requirement of gas recycle compressor; membrane surface is suitable for immobilization of active SRB, resulting in the formation of biofilms, thus preventing washout problems associated with suspended culture reactors; and lower operating costs in membrane bioreactors, eliminating gas recompression and gas recycle costs. Information is provided on sulfate reduction rate studies and on biokinetic tests with suspended SRB in anaerobic digester sludge and sediment master culture reactors and with SRB biofilms in bench-scale SRB membrane bioreactors. Biokinetic parameters have been determined using biokinetic models for the master culture and membrane bioreactor systems. Data are presented on the effect of acid mine water sulfate loading at 25, 50, 75 and 100 ml/min in scale-up SRB membrane units, under varied temperatures (25, 35 and 40 °C) to determine and optimize sulfate conversions for an effective AMD biotreatment. Pilot-scale studies have generated data on the effect of flow rates of acid mine water (MGD) and varied inlet sulfate concentrations in the influents on the resultant outlet sulfate concentration in the effluents and on the number of SRB membrane modules needed for the desired sulfate conversion in those systems. The pilot-scale data indicate that the SRB membrane bioreactors systems can be applied toward field-scale biotreatment of AMD and for recovery of high purity metals and an agriculturally usable water.
Similar content being viewed by others
References
Abram JW & Nedwell DB (1978) Inhibition of methanogenesis by sulphate reducing bacteria competing for transferred hydrogen. Arch. Microbiol. 117: 89–92
Badzioug W & Thauer RK (1978) Growth yields and growth rates of Desulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate and hydrogen plus thiosulfate as sole energy sources. Arch. Microbiol. 117: 209214
Battaglia-Brunet F, Foucher S, Ignatiadis I & Morin D. (2000) Production of H2S by sulfate reducing bacteria in a two-column gas/liquid reactor for the purification of metal-containing effluents. XXI IMPC. Roma, 2327 July 2000, pp. B 129/17
Battaglia-Brunet F, Foucher S, Denamur A. Ignatiadis I, Michel C & Morin D (2002) Reduction of chromate by fixed films of sulfatereducing bacteria using hydrogen as an electron source. J. Indust. Microbiol. Biotechnol. 28: 154–159
Brandis A & Thauer R (1981) Growth of Desulfovibrio species on hydrogen and sulfate as sole energy source. J. Gen. Microbiol. 126: 249–252
Brandt KK, Vester F, Jensen AN & Ingvorsen K (2001) Sulfate reduction dynamics and enumeration of sulfate reducing bacteria in hypersaline sediments of the Great Salt Lake (Utah, USA). Microb. Ecol. 41: 1–11
Brindle K & Stephenson T (1996) The application of membrane biological reactors for the treatment of wastewaters. Biotechnol. Bioeng. 44: 596–594
Buisman CJN, Uspeert P, Hol A, Janssen AJH, tenHagen R & Lettinga G (1991) Kinetic parameters of a n-fixed culture oxidizing sulfide and sulfur with oxygen. Biotechnol. Bioeng. 38: 813–820
Choo KH & Lee CH (1996) Membrane fouling microorganisms in the membrane-coupled anaerobic bioreactor. Wat. Res. 30: 1771–1780
Cote P, Bersillon JL, Huyard A & Faup G (1988) Bubble-free aeration using membrane process analysis. J.Wat. Pollut. Contr. Fed. 60: 1986–1992
Du Preez LA, Odendaal JP, Maree JP & Pansenby M (1992) Biological removal of sulfate from industrial effluents using producer gas as energy source. Environ. Technol. 13: 875–882
Dvorak DH, Hedin RS, Edenborn HM & McIntire PE (1992) Treatment of metal contaminated water using bacterial sulfate reduction: Results from pilot scale reactors. Biotechnol. Bioengr. 40: 609–616
Federovich V, Greben M, Kalyuzbnyi S, Lens P & Hulshoff Pol LW (2000) Use of hydrophobic membranes to supply hydrogen to sulfate reducing bioreactors. Biodegradation 11: 295–303
Foucher S, Battaglia-Brunet A, Ignatiadis I & Mpron D (2001) Treatment by sulfate-reducing bacteria of Chessy acid-mine drainage and metals recovery. Chem. Bioengr. Sci. 56: 1639–1645
Ghyoot WR & Verstraete WH ( 1997) Coupling membrane filtration to anaerobic primary sludge digestion. Environ. Technol. 18: 569–580
Govind R, Rao P & Tabak HH (2000) Membrane reactor studies for treatment of acid mine drainage. U.S. EPA Report, National Risk Management Research Laboratory, ORD, Cincinnati, OH, 30 September 2000, pp.85
Greben M (1999) Biological sulfate reduction in a membrane bioreactor. Ms. Thesis Wageningen Agricultural University, Wageningen, The Netherlands
Holder EL & Tabak HH (2002) Use of respirometry to measure hydrogen consumption by sulfate reducing bacteria in the presence of toxic metals. SETAC 23rd Annual Meeting in North America, Salt Lake City, Utah, USA, 16–20 November 2002
Hulshoff Pol L, Lens P, Stains AJM & Lettinga G (1998) Anaerobic treatment of sulfaterich wastewaters. Biodegradation 9: 213–224
Ingvorsen K & Jorgensen BB (1984) Kinetics of sulfate uptake by freshwater and marine species of Desulfovibrio. Arch. Microbiol. 139: 61–68
Ingvorsen K, Zehnder AJB & Jorgensen BB (1984) Kinetics of sulfate and acetate uptake by Desulfovibrio postgatei. Appl. Environ. Microbiol. 47(1): 403–408
Ito T, Okabe S, Satoh H & Watanabe Y (2002) Successional development of sulfate reducing bacterial populations and their activities in a wastewater biofilm growing under microaerophilic conditions. Appl. Environ. Microbiol. 68(3): 1392–1402
Kalyuzhnyi S and Fedorowich V (1997) Integrated mathematical model of UASB reactor for competition between sulfate reduction and methanogenesis. Wat. Sci. Technol. 36(6–7): 201–208
Lovley DR, Dwyer D & Klug MJ (1982) Kinetic analysis of competition between sulfate reducers and methanogenesis for hydrogen in sediments. Appl. Environ. Microbiol. 43: 1373–1379
Lupton FS, Conrad R & Zeikus JG (1984) Physiological function of hydrogen metabolism during growth of sulfidogenic bacteria on organic substrates. J. Bacteriol. 159(3): 843–849
Lupton FS & Zeikus JG (1984) Physiological basis for sulfatedependent hydrogen competition between sulfidogens and methanogens. Curr. Microbiol. 11: 7–12.
Maree JP & Hill E (1989) Biological removal of sulfate from industrial effluents and concomitant production of sulfur. Wat. Sci. Tech 21: 265276
Mizuno O, Takagi H & Noike T (1998) Biological sulfate removal in an acetogenic bioreactor with an ultrafiltration membrane system. Wat. Sci. Tech. 38(4/5): 513–520
Nanninga HJ & Gottschal (1987) Properties of Desulfovibrio carbonicolicus sp. Nov. and other sulfate-reducing bacteria isolated from an anaerobic-purification plant. Appl. Environ. Microbiol. 53(3): 802–809
Nielsen PH (1987) Biofilm dynamics and kinetics during highrate sulfate reduction under anaerobic conditions. Appl. Environ. Microbial. 53: 27–32
Noguera DR, Brusseau GA & Rittmann B & Stahl DA (1998) A unified model describing the role of hydrogen in the growth of Desulfovibrio vulgaris under different environmental conditions. Biotechnol. Bioengr. 59(6): 732–746
Odom JM & Peck Jr. HD (1981) Hydrogen cycling as a general mechanism for energy coupling in the sulfate-reducing bacteria, Desulfovibrio sp. FEMS Microbiol. Lett. 12: 47–50
Okabe, S. Nielsen PH, Jones WL & Charaklis WG (1995) Rate and Stoichiometry of microbial sulfate reduction by Desulfovibrio desulfuricans in biofilms. Biofouling 9: 63–83
Okabe S, Itoh, T, Satob H & Watanabe Y (1999) Analyses of spatial distribution of sulfate reducing bacteria and their activity in aerobic wastewater biofilms. Appl. Environ. Microbiol. 65: 5107–5116
Pankhania M, Stephenson T & Semmens MJ (1994) Hollow fibre bioreactor for wastewater using bubbleless membrane aeration. Wat. Res. 28:10 2233–2236
Paques Biosystems BV PO Box 52, 8560 AB Balk, The Netherlands (2000) Bioprocess Technology: Sulfate removal and metal recovery; Heavy metals removal; Hydrogen sulfide removal. Pamphlets and pers. comm.
Phelps TJ, Conrad R and Zeikus JG (1985) Sulfate-dependent interspecies hydrogen transfer between Methanosarcina barkeri and Desulfovibrio vulgaris during coculture metabolism of acetate and methanol. Appl. Environ. Microbiol. 50(3): 583–594
Ramsing NB, Kuhl M & Jorgensen BB ( 1993) Distribution of sulfate reducing bacteria, 02 and H2S in photosynthetic biofilms determined by oligonucleotide probes and microelectrodes. Appl. Environ. Microbiol. 59: 3840–3849
Rinzema A & Lettinga G (1988) Anaerobic treatment of sulfate containing wastewater. In: Wise DL (Ed) Biotreatment Systems 3, CRC Press, Boca Raton, FL pp. 65–109
Robinson JA & Tiedje JM (1984) Competition between sulfatereducing and methanogenic bacteria for hydrogen under resting and growing conditions. Arch. Microbiol. 137: 26–32
Ruthemond C, Camper A & Wilderer PA (1994) Biofilms growing on gas permeable membranes. Wat. Sci. Tech. 29: 11447–11454
Santegoeds CM, Ferdelman TG, Muyzer G & de Beer D (1998) Structural and functional dynamics of sulfate reducing populations in bacterial biofilms. Appl. Environ. Microbiol. 64: 3731–3739
Shimada K (1987) Removal of heavy metals from mine wastewater using sulfate reducing bacteria. J.Wat.Wastewat. Jpn. 31: 52–56
Sipma J, Lens P, Vieira A, Miron Y, Van Lier JB, Hulshoff Pol LW & Lettinga G (1999) Thermophilic sulphate reduction in upflow anaerobic sludge bed reactors under acidifying conditions. Proc. Biochem. 35(5): 509–522
Sorensen J, Christensen D & Jorgensen BB (1981) Volatile fatty acids and hydrogen as substrates for sulfate-reducing bacteria in anaerobic marine sediment. Appl. Environ. Microbiol. 42(1): 5–11
Traore AS, Fardeau M-L, Hatchikian CE, LeGall J & Belaich JP (1983) Energetics of growth of a defined mixed culture of Desulfovibrio vulgaris and Methanosarcina barkeri: Intraspecies hydrogen transfer in batch and continuous cultures. Appl. Environ. Microbiol. 46(5): 1152–1156
Van Houten RT, Van der Spoul H, Van Aelst AC, Hulshoff Pol, LW & Lettinga G (1996) Biological sulphate reduction using synthesis gas. Biotechnol. Bioengr. 50: 136–144
Vick RTB, Blanch HW & Wilke CR (1983) Microbial hollow fiber bioreactors. Trends in Biotechnol. 1: 135–139
Weijma J, Hulshoff Pol LW, Stams AJM & Lettinga G (2000) Performance of a thermophilic sulfate and sulfite reducing high rate anaerobic reactor fed with methanol. Biodegradation 11(6): 429–439
Widdel F & Pfennig N (1982) Studies on dissimilatory sulfatereducing bacteria that decompose fatty acids. II. Incomplete oxidation of propionate by Desulfovibrio propionicus gen. nov., sp, nov. Arch. Microbiol. 131: 360–365.
Widdel F & Bak F (1991) Gram-negative mesophilic sulfatereducing bacteria. In: Balows A, Truper HG, Dworkin M, Harder W & Schleifer KH (Eds). The Prokaryotes. 2nd edn. Springer-Verlag, New York. pp. 3352–3378.
Widdel F & Hansen TA (1991) The dissimilatory sulfate and sulfur-reducing bacteria. In Balows A, Truper HG, Dworkin M, Harderand W & Scleider K-H (Eds) The Prokaryotes, 2nd edn. (pp. 583–624). Springer-Verlag, New York.
Yamamoto K, Hiasa M, Mahmood T & Matsuo T (1989) Direct solid-liquid separation using hollow fiber membrane in an activated sludge aeration tank. Wat. Sci. Technol. 21: 43–49.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tabak, H.H., Govind, R. Advances In Biotreatment of Acid Mine Drainage and Biorecovery of Metals: 2. Membrane Bioreactor System for Sulfate Reduction. Biodegradation 14, 437–452 (2003). https://doi.org/10.1023/A:1027332918844
Issue Date:
DOI: https://doi.org/10.1023/A:1027332918844