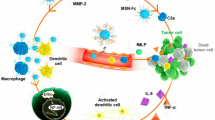
Abstract
Cancer represents a significant cause of morbidity and mortality. Definitive chemotherapy, surgery and radiotherapy treatment have not improved the “5-year survival period” and have shown recurrence. Currently, cancer immunotherapy is reported to be a promising therapeutic modality that aims to potentiate immune response against cancer by employing immune checkpoint inhibitors, cancer vaccines and immunomodulators. Inhibition of immune checkpoints such as PD-1/PDL1, CTLA and TIM molecules using monoclonal antibodies, ligands or both are proven to be the most successful anticancer immunotherapy. But the application of immunotherapy involves critical challenges such as non-responsiveness and systemic toxicity due to the administration of high dose. To mitigate the above challenges, nanomaterial-based delivery and therapy have been adopted to inhibit the immune checkpoints and induce an anticancer immune response. Specifically, mesoporous silica-based materials for cancer therapy are shown to be versatile materials for the above purpose. Mesoporous silica nanoparticle (MSN) based cancer immunotherapy overcomes numerous challenges and offers novel strategies for improving conventional immunotherapies. MSN has a high surface area, porosity and biocompatibility; it also has natural immune-adjuvant properties, which have been reported to be the best candidate material for immunotherapeutic delivery. This review will focus on the use of MSN as carriers for delivering immune checkpoint inhibitors and their efficacy in cancer combination therapy.






Similar content being viewed by others
References
Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3-23. https://doi.org/10.1016/j.jaci.2009.12.980.
Guermonprez P, Valladeau J, Zitvogel L, et al. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20(1):621–67. https://doi.org/10.1146/annurev.immunol.20.100301.064828.
Burger D, Dayer JM. Cytokines, acute‐phase proteins, and hormones: IL‐1 and TNF‐α production in contact‐mediated activation of monocytes by T lymphocytes. Annals of the New York Academy of Sciences. 2002;966(1):464–73. https://doi.org/10.1111/j.1749-6632.2002.tb04248.x.
Tian T, Olson S, Whitacre JM, et al. The origins of cancer robustness and evolvability. Integr Biol (Camb). 2011;3(1):17–30. https://doi.org/10.1039/c0ib00046a.
Boon T, Cerottini JC, Van den Eynde B, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. https://doi.org/10.1146/annurev.iy.12.040194.002005.
Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8. https://doi.org/10.1038/ni1102-991.
Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–7. https://doi.org/10.1158/1078-0432.CCR-12-1362.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1. https://doi.org/10.1016/j.immuni.2013.07.012.
Xu Z, Zhen B, Park Y, et al. Designing therapeutic cancer vaccine trials with delayed treatment effect. Stat Med. 2017;36(4):592–605. https://doi.org/10.1002/sim.7157.
Varadé J, Magadán S, González-Fernández Á. Human immunology and immunotherapy: main achievements and challenges. Cell Mol Immunol. 2021;18(4):805–28. https://doi.org/10.1038/s41423-020-00530-6.
Chabalgoity JA, Dougan G, Mastroeni P, et al. Live bacteria as the basis for immunotherapies against cancer. Expert Rev Vaccines. 2002;1(4):495–505. https://doi.org/10.1586/14760584.1.4.495.
Davola ME, Mossman KL. Oncolytic viruses: how “lytic” must they be for therapeutic efficacy? Oncoimmunology. 2019;8(6):e1581528. https://doi.org/10.1080/2162402X.2019.1596006.
Fu C, Ma T, Zhou L, et al. Dendritic cell-based vaccines against cancer: challenges, advances and future opportunities. Immunol Invest. 2022;51(8):2133–58. https://doi.org/10.1080/08820139.2022.2109486.
Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. The Lancet. 2009;373(9668):1033–40. https://doi.org/10.1007/s11033-018-4427-x.
Zhang WG, Liu SH, Cao XM, et al. A phase-I clinical trial of active immunotherapy for acute leukemia using inactivated autologous leukemia cells mixed with IL-2, GM-CSF, and IL-6. Leuk Res. 2005;29(1):3–9. https://doi.org/10.1016/j.leukres.2004.04.015.
Tanaka F, Hashimoto W, Okamura H, et al. Rapid generation of potent and tumor-specific cytotoxic T lymphocytes by interleukin 18 using dendritic cells and natural killer cells. Can Res. 2000;60(17):4838–44.
Hosseinkhani N, Derakhshani A, Kooshkaki O, et al. Immune checkpoints and CAR-T cells: the pioneers in future cancer therapies? Int J Mol Sci. 2020;21(21):8305. https://doi.org/10.3390/ijms21218305.
Webster RM. The immune checkpoint inhibitors: where are we now? Nat Rev Drug Discov. 2014;13(12):883–4. https://doi.org/10.1038/nrd4476.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. https://doi.org/10.1038/nrc3239.
Harding FA, McArthur JG, Gross JA, et al. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356(6370):607–9. https://doi.org/10.1038/356607a0.
Dong C, Juedes AE, Temann UA, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409(6816):97–101. https://doi.org/10.1038/35051100.
Kaleeba JA, Offner H, Vandenbark AA, et al. The OX-40 receptor provides a potent co-stimulatory signal capable of inducing encephalitogenicity in myelin-specific CD4+ T cells. Int Immunol. 1998;10(4):453–61. https://doi.org/10.1093/intimm/10.4.453.
Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Semin Immunol. 2004;16(3):185–96. https://doi.org/10.1016/j.smim.2004.02.006.
Villanueva MT. Cancer immunotherapy: searching in the immune checkpoint black box. Nat Rev Drug Discov. 2017;16(9):599. https://doi.org/10.1038/nrd.2017.163.
Barbee MS, Ogunniyi A, Horvat TZ, et al. Current status and future directions of the immune checkpoint inhibitors ipilimumab, pembrolizumab, and nivolumab in oncology. Ann Pharmacother. 2015;49(8):907–37. https://doi.org/10.1177/1060028015586218.
Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–76. https://doi.org/10.1007/s10549-013-2581-3.
Hassounah NB, Malladi VS, Huang Y, et al. Identification and characterization of an alternative cancer-derived PD-L1 splice variant. Cancer Immunol Immunother. 2019;68(3):407–20. https://doi.org/10.1007/s00262-018-2284-z.
Thumar JR, Kluger HM. Ipilimumab: a promising immunotherapy for melanoma. Oncology. 2010;24(14):1280.
Helmy KY, Patel SA, Nahas GR, et al. Cancer immunotherapy: accomplishments to date and future promise. Ther Deliv. 2013;4(10):1307–20. https://doi.org/10.4155/tde.13.88.
Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Anderson AC. Tim-3, a negative regulator of anti-tumor immunity. Curr Opin Immunol. 2012;24(2):213–6. https://doi.org/10.1016/j.coi.2011.12.005.
Dougall WC, Kurtulus S, Smyth MJ, et al. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol Rev. 2017;276(1):112–20. https://doi.org/10.1111/imr.12518.
Rosenholm JM, Mamaeva V, Sahlgren C, et al. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine. 2012;7(1):111–20. https://doi.org/10.2217/nnm.11.166.
Ordered porous materials for emerging applications. Nature. 2002;417(6891):813–21. https://doi.org/10.1038/nature00785.
Wan Y, Zhang D, Hao N, et al. Organic groups functionalised mesoporous silicates. Int J Nanotechnol. 2007;4(1–2):66–99.
Kuthati Y, Sung PJ, Weng CF, et al. Functionalization of mesoporous silica nanoparticles for targeting, biocompatibility, combined cancer therapies and theragnosis. J Nanosci Nanotechnol. 2013;13(4):2399–430. https://doi.org/10.1166/jnn.2013.7363.
An M, Li M, Xi J, et al. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Interfaces. 2017;9(28):23466–75. https://doi.org/10.1021/acsami.7b06024.
Wang Y, Zhao Q, Han N, et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine: Nanotechnol Biol Med. 2015;11(2):313–27. https://doi.org/10.1016/j.nano.2014.09.014.
Singh LP, Bhattacharyya SK, Kumar R, et al. Sol-Gel processing of silica nanoparticles and their applications. Adv Coll Interface Sci. 2014;214:17–37. https://doi.org/10.1016/j.cis.2014.10.007.
Bolla PA, Huggias S, Serradell MA, et al. Synthesis and catalytic application of silver nanoparticles supported on Lactobacillus kefiri S-layer proteins. Nanomaterials. 2020;10(11):2322. https://doi.org/10.3390/nano10112322.
Deodhar GV, Adams ML, Trewyn BG. Controlled release and intracellular protein delivery from mesoporous silica nanoparticles. Biotechnol J. 2017;12(1):1600408. https://doi.org/10.1002/biot.201600408.
Yang YW. Towards biocompatible nanovalves based on mesoporous silica nanoparticles. MedChemComm. 2011;2(11):1033–49.
Trewyn BG, Slowing II, Giri S, et al. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc Chem Res. 2007;40(9):846–53. https://doi.org/10.1021/ar600032u.
Gu J, Su S, Zhu M, et al. Targeted doxorubicin delivery to liver cancer cells by PEGylated mesoporous silica nanoparticles with a pH-dependent release profile. Microporous Mesoporous Mater. 2012;161:160–7. https://doi.org/10.1016/j.micromeso.2012.05.035.
Qi X, Yu D, Jia B, et al. Targeting CD133+ laryngeal carcinoma cells with chemotherapeutic drugs and siRNA against ABCG2 mediated by thermo/pH-sensitive mesoporous silica nanoparticles. Tumor biology. 2016;37:2209–17. https://doi.org/10.1007/s13277-015-4007-9.
Zhang H, Zhang W, Zhou Y, et al. Dual functional mesoporous silicon nanoparticles enhance the radiosensitivity of VPA in glioblastoma. Transl Oncol. 2017;10(2):229–40. https://doi.org/10.1016/j.tranon.2016.12.011.
Chen X, Sun H, Hu J, et al. Transferrin gated mesoporous silica nanoparticles for redox-responsive and targeted drug delivery. Colloids Surf, B. 2017;152:77–84. https://doi.org/10.1016/j.colsurfb.2017.01.010.
Sweeney SK, Luo Y, O’Donnell MA, et al. Peptide-mediated targeting mesoporous silica nanoparticles: a novel tool for fighting bladder cancer. J Biomed Nanotechnol. 2017;13(2):232–42. https://doi.org/10.1166/jbn.2017.2339.
Hirano Y, Kando Y, Hayashi T, et al. Synthesis and cell attachment activity of bioactive oligopeptides: RGD, RGDS, RGDV, and RGDT. J Biomed Mater Res. 1991;25(12):1523–34. https://doi.org/10.1002/jbm.820251209.
Babaei M, Abnous K, Taghdisi SM, et al. Synthesis of theranostic epithelial cell adhesion molecule targeted mesoporous silica nanoparticle with gold gatekeeper for hepatocellular carcinoma. Nanomedicine. 2017;12(11):1261–79. https://doi.org/10.1002/jbm.820251209.
Zhou S, Wu D, Yin X, et al. Intracellular pH-responsive and rituximab-conjugated mesoporous silica nanoparticles for targeted drug delivery to lymphoma B cells. J Exp Clin Cancer Res. 2017;36:1–4. https://doi.org/10.1186/s13046-017-0492-6.
Heinemann S, Coradin T, Desimone MF. Bio-inspired silica–collagen materials: applications and perspectives in the medical field. Biomater Sci. 2013;1(7):688–702. https://doi.org/10.1039/c3bm00014a.
He Q, Zhang Z, Gao F, et al. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 2011;7(2):271–80. https://doi.org/10.1002/smll.201001459.
Kuang Y, Zhai J, Xiao Q, et al. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: a review. Int J Biol Macromol. 2021;193:457–73. https://doi.org/10.1016/j.ijbiomac.2021.10.142.
Mamaeva V, Sahlgren C, Lindén M. Mesoporous silica nanoparticles in medicine—recent advances. Adv Drug Del Rev. 2013;65(5):689–702. https://doi.org/10.1016/j.addr.2012.07.018
Li Z, Zhang Y, Feng N. Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin Drug Deliv. 2019;16(3):219–37. https://doi.org/10.1080/17425247.2019.1575806.
Fu C, Liu T, Li L, et al. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials. 2013;34(10):2565–75. https://doi.org/10.1016/j.biomaterials.2012.12.043.
He Q, Zhang Z, Gao F, et al. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 2011;7(2):271–80. https://doi.org/10.1002/smll.201001459.
Lu J, Liong M, Li Z, et al. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6(16):1794–805. https://doi.org/10.1002/smll.201000538.
Yu T, Greish K, McGill LD, et al. Influence of geometry, porosity, and surface characteristics of silica nanoparticles on acute toxicity: their vasculature effect and tolerance threshold. ACS Nano. 2012;6(3):2289–301. https://doi.org/10.1021/nn2043803.
Nguyen TL, Choi Y, Kim J. Mesoporous silica as a versatile platform for cancer immunotherapy. Adv Mater. 2019;31(34):1803953. https://doi.org/10.1002/adma.201803953.
Chen Y, Chen H, Zeng D, et al. Core/shell structured hollow mesoporous nanocapsules: a potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano. 2010;4(10):6001–13. https://doi.org/10.1021/nn1015117.
Liu Q, Zhang J, Xia W, et al. Magnetic field enhanced cell uptake efficiency of magnetic silica mesoporous nanoparticles. Nanoscale. 2012;4(11):3415–21. https://doi.org/10.1039/c2nr30352c.
Hao N, Li L, Tang F. Shape matters when engineering mesoporous silica-based nanomedicines. Biomater Sci. 2016;4(4):575–91. https://doi.org/10.1039/c5bm00589b.
Jiang W, Kim BY, Rutka JT, et al. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotechnol. 2008;3(3):145–50. https://doi.org/10.1038/nnano.2008.30.
Vivero-Escoto JL, Slowing II, Trewyn BG, et al. Mesoporous silica nanoparticles for intracellular controlled drug delivery. Small. 2010;6(18):1952–67. https://doi.org/10.1002/smll.200901789.
Douroumis D, Onyesom I, Maniruzzaman M, et al. Mesoporous silica nanoparticles in nanotechnology. Crit Rev Biotechnol. 2013;33(3):229–45. https://doi.org/10.3109/07388551.2012.685860.
Song Y, Li Y, Xu Q, et al. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: advances, challenges, and outlook. Int J Nanomed. 2017;12:87. https://doi.org/10.2147/IJN.S117495.
Mamaeva V, Sahlgren C, Lindén M. Mesoporous silica nanoparticles in medicine-recent advances. Adv Drug Deliv Rev. 2013;65(5):689–702. https://doi.org/10.1016/j.addr.2012.07.018.
He Q, Shi J. MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Adv Mater. 2014;26(3):391–411. https://doi.org/10.1002/adma.201303123.
Wen J, Yang K, Liu F, et al. Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems. Chem Soc Rev. 2017;46(19):6024–45. https://doi.org/10.1039/c7cs00219j.
Zheng DW, Chen JL, Zhu JY, et al. Highly integrated nano-platform for breaking the barrier between chemotherapy and immunotherapy. Nano Lett. 2016;16(7):4341–7. https://doi.org/10.1021/acs.nanolett.6b01432.
Wang X, Li X, Yoshiyuki K, et al. Erratum to supporting information of comprehensive mechanism analysis of mesoporous-silica-nanoparticle-induced cancer immunotherapy. Adv Healthc Mater. 2019;8(23): e1901432. https://doi.org/10.1002/adhm.201901432.
Heidegger S, Gößl D, Schmidt A, et al. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale. 2016;8(2):938–48. https://doi.org/10.1039/c5nr06122a.
Hao N, Liu H, Li L, Chen D, Li L, Tang F. In vitro degradation behavior of silica nanoparticles under physiological conditions. J Nanosci Nanotechnol. 2012;12(8):6346–54. https://doi.org/10.1166/jnn.2012.6199.
Fukushima H, Turkbey B, Pinto PA, et al. Near-infrared photoimmunotherapy (NIR-PIT) in urologic cancers. Cancers (Basel). 2022;14(12):2996. https://doi.org/10.3390/cancers14122996.
Li B, Zhang X, Wu Z, et al. Reducing postoperative recurrence of early-stage hepatocellular carcinoma by a wound-targeted nanodrug. Adv Sci (Weinh). 2022;9(20):e2200477. https://doi.org/10.1002/advs.202200477.
Peng H, Shen J, Long X, et al. Local Release of TGF-β Inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv Sci. 2022;9(10):2105240. https://doi.org/10.1002/advs.202105240.
Allen SD, Liu X, Jiang J, et al. Immune checkpoint inhibition in syngeneic mouse cancer models by a silicasome nanocarrier delivering a GSK3 inhibitor. Biomaterials. 2021;269:120635. https://doi.org/10.1016/j.biomaterials.2020.120635.
He Z, Zhang H, Li H, et al. Preparation, biosafety, and cytotoxicity studies of a newly tumor-microenvironment-responsive biodegradable mesoporous silica nanosystem based on multimodal and synergistic treatment. Oxid Med Cell Longev. 2020;2020. https://doi.org/10.1155/2020/7152173.
Shao D, Zhang F, Chen F, et al. Biomimetic diselenide-bridged mesoporous organosilica nanoparticles as an x-ray-responsive biodegradable carrier for chemo-immunotherapy. Adv Mater. 2020;32(50). https://doi.org/10.1002/adma.202004385
Eleftheriadis T, Pissas G, Zarogiannis S, et al. Crystalline silica activates the T-cell and the B-cell antigen receptor complexes and induces T-cell and B-cell proliferation. Autoimmunity. 2019;52(3):136–43. https://doi.org/10.1080/08916934.2019.1614171.
Sun Z, Wang Z, Wang T, et al. Biodegradable MnO-based nanoparticles with engineering surface for tumor therapy: simultaneous fenton-like ion delivery and immune activation. ACS Nano. 2022. https://doi.org/10.1021/acsnano.2c00969.
Peng H, Shen J, Long X, et al. Local release of TGF-β inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv Sci (Weinh). 2022;9(10):e2105240. https://doi.org/10.1002/advs.202105240.
Zhao P, Xu Y, Ji W, et al. Hybrid membrane nanovaccines combined with immune checkpoint blockade to enhance cancer immunotherapy. Int J Nanomedicine [Internet]. 2022;17:73–89. https://doi.org/10.2147/IJN.S346044.
Huang C, Mendez N, Echeagaray OH, et al. Immunostimulatory TLR7 agonist-nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Adv Ther (Weinh). 2020;3(6):1900200. https://doi.org/10.1002/adtp.201900200.
Choi B, Jung H, Yu B, et al. Sequential MR image-guided local immune checkpoint blockade cancer immunotherapy using ferumoxytol capped ultralarge pore mesoporous silica carriers after standard chemotherapy. Small. 2019;15(52). https://doi.org/10.1002/smll.201904378.
Haber T, Cornejo YR, Aramburo S, et al. Specific targeting of ovarian tumor-associated macrophages by large, anionic nanoparticles. Proc Natl Acad Sci. 2020;117(33):19737–45. https://doi.org/10.1073/pnas.1917424117.
Shahidi M, Abazari O, Dayati P, et al. Multicomponent siRNA/miRNA-loaded modified mesoporous silica nanoparticles targeted bladder cancer for a highly effective combination therapy. Frontiers in bioengineering and biotechnology. 2022;10. https://doi.org/10.3389/fbioe.2022.949704.
Ma H, Ma Z, Chen Q, et al. Bifunctional, copper-doped, mesoporous silica nanosphere-modified, bioceramic scaffolds for bone tumor therapy. Front Chem. 2020;8:610232. https://doi.org/10.3389/fchem.2020.610232.
Wang Z, Chen L, Ma Y, et al. Peptide vaccine-conjugated mesoporous carriers synergize with immunogenic cell death and PD-L1 blockade for amplified immunotherapy of metastatic spinal. J Nanobiotechnol. 2021;19(1). https://doi.org/10.1186/s12951-021-00975-5.
Sun M, Gu P, Yang Y, et al. Mesoporous silica nanoparticles inflame tumors to overcome anti-PD-1 resistance through TLR4-NFκB axis. J Immunother Cancer. 2021;9(6). https://doi.org/10.1136/jitc-2021-002508.
Yu X, Wang X, Yamazaki A, et al. Tumor microenvironment-regulated nanoplatforms for the inhibition of tumor growth and metastasis in chemo-immunotherapy. J Mater Chem B. 2022;10(19). https://doi.org/10.1039/d2tb00337f.
Wang X, Li X, Ito A, et al. Synergistic anti-tumor efficacy of a hollow mesoporous silica-based cancer vaccine and an immune checkpoint inhibitor at the local site. Acta Biomater. 2022;145:235–45. https://doi.org/10.1016/j.actbio.2022.04.001.
Li X, Wang X, Ito A, et al. A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nat Commun. 2020;11(1). https://doi.org/10.1038/s41467-020-17637-z.
Nguyen TL, Cha BG, Choi Y, et al. Injectable dual-scale mesoporous silica cancer vaccine enabling efficient delivery of antigen/adjuvant-loaded nanoparticles to dendritic cells recruited in local macroporous scaffold. Biomaterials. 2020;239. https://doi.org/10.1016/j.biomaterials.2020.119859.
Kim H, Yuk SA, Dieterly AM, et al. Nanosac, a noncationic and soft polyphenol nanocapsule, enables systemic delivery of siRNA to solid tumors. ACS Nano. 2021;15(3):4576–93. https://doi.org/10.1021/acsnano.0c08694.
Im S, Lee J, Park D, et al. Hypoxia-triggered transforming immunomodulator for cancer immunotherapy via photodynamically enhanced antigen presentation of dendritic cell. ACS Nano. 2018;13(1):476–88. https://doi.org/10.1021/acsnano.8b07045.
Ding B, Shao S, Yu C, et al. Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv Mater. 2018;30(52):1802479. https://doi.org/10.1002/adma.201802479.
Xu C, Nam J, Hong H, et al. Positron emission tomography-guided photodynamic therapy with biodegradable mesoporous silica nanoparticles for personalized cancer immunotherapy. ACS Nano. 2019;13(10):12148–61. https://doi.org/10.1021/acsnano.9b06691.
Yang G, Xu L, Xu J, et al. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018;18(4):2475–84. https://doi.org/10.1021/acs.nanolett.8b00040.
Terracciano M, Fontana F, Falanga AP, et al. Development of surface chemical strategies for synthesizing redox-responsive diatomite nanoparticles as a green platform for on-demand intracellular release of an antisense peptide nucleic acid anticancer agent. Small. 2022;18(41):e2204732. https://doi.org/10.1002/smll.202204732.
Yang Y, Chen F, Xu N, et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials. 2022;281. https://doi.org/10.1016/j.biomaterials.2022.121368.
Feng Y, Xie X, Zhang H, et al. Multistage-responsive nanovehicle to improve tumor penetration for dual-modality imaging-guided photodynamic-immunotherapy. Biomaterials. 2021;275:120990. https://doi.org/10.1016/j.biomaterials.2021.120990.
Chen Y, Ma H, Wang W, et al. A size-tunable nanoplatform: enhanced MMP2-activated chemo-photodynamic immunotherapy based on biodegradable mesoporous silica nanoparticles. Biomater Sci. 2021;9(3):917–29. https://doi.org/10.1039/d0bm01452d.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashitha, K.C., M, G., N.R, S. et al. Leveraging mesoporous silica nanomaterial for optimal immunotherapeutics against cancer. In vitro models 2, 153–169 (2023). https://doi.org/10.1007/s44164-023-00061-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44164-023-00061-0