Abstract
Background
Photobiomodulation therapy (PBMT), due to its anti-inflammatory, analgesic effects, and most importantly as a non-invasive procedure, has currently gained a special setting in pain relief and the treatment of Spinal cord injuries (SCI). However, the mechanism of action of the PBM is not yet completely understood.
Methods
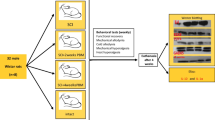
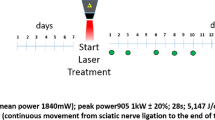
In this study, SCI is induced by an aneurysm clip, and PBM therapy was applied by a continuous-wave (CW) laser with a wavelength of 660 nm. Adult male rats were divided into four groups: Control, SCI, SCI + PBMT 90s, and SCI + PBMT 117s. After 7 weeks, hyperalgesia, allodynia, and functional recovery were assessed. Fibroblasts infiltrating the spinal cord were counted after H&E staining. The expression of epigenetic factors (HDAC2, DNMT3a), protein relevant for pain (GAD65), and astrocytes marker (GFAP) after 4 weeks of daily PBMT (90 and 117s) was probed by western blotting.
Results
Both PBMTs (90 and 117s) significantly improved the pain and ability to move and fibroblast invasion was reduced. SCI + PBMT 90s, increased GAD65, HDAC2, and DNMT3a expression. However, PBMT 117s decreased GFAP, HDAC2, and DNMT3a.
Conclusion
PBMT 90 and 117s improved the pain, and functional recovery equally. The regulation of epigenetic mechanisms appears to be a significant effect of PBMT117s, which emphasizes on impact of radiation duration and accumulative energy.
Graphical abstract





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Leibinger, M., et al. (2019). GSK3-CRMP2 signaling mediates axonal regeneration induced by Pten knockout. Communications Biology, 2(1), 1–13.
Chambel, S. S., Tavares, I., & Cruz, C. D. (2020). Chronic pain after spinal cord injury: Is there a role for neuron-immune dysregulation? Frontiers in Physiology, 11, 748.
Van Gorp, S., et al. (2015). Pain prevalence and its determinants after spinal cord injury: A systematic review. European Journal of Pain, 19(1), 5–14.
Hagen, E. M., & Rekand, T. (2015). Management of neuropathic pain associated with spinal cord injury. Pain and therapy, 4(1), 51–65.
Austin, P. J., & Moalem-Taylor, G. (2010). The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. Journal of Neuroimmunology, 229(1–2), 26–50.
Zhao, H., et al. (2017). The role of microglia in the pathobiology of neuropathic pain development: what do we know? BJA: British Journal of Anaesthesia, 118(4), 504–516.
Descalzi, G., et al. (2015). Epigenetic mechanisms of chronic pain. Trends in Neurosciences, 38(4), 237–246.
Luo, D., et al. (2021). Epigenetic modifications in neuropathic pain. Molecular Pain, 17, 17448069211056768.
Moore, L. D., Le, T., & Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology, 38(1), 23–38.
Wang, Y., et al. (2011). Intrathecal 5-azacytidine inhibits global DNA methylation and methyl-CpG-binding protein 2 expression and alleviates neuropathic pain in rats following chronic constriction injury. Brain Research, 1418, 64–69.
Tochiki, K. K., et al. (2012). The expression of spinal methyl-CpG-binding protein 2, DNA methyltransferases and histone deacetylases is modulated in persistent pain states. Molecular Pain. https://doi.org/10.1186/1744-8069-8-14
Liang, L., & Tao, Y.-X. (2018). Expression of acetyl-histone H3 and acetyl-histone H4 in dorsal root ganglion and spinal dorsal horn in rat chronic pain models. Life Sciences, 211, 182–188.
Li, K., et al. (2014). Epigenetic upregulation of Cdk5 in the dorsal horn contributes to neuropathic pain in rats. NeuroReport, 25(14), 1116–1121.
Cherng, C.-H., et al. (2014). Baicalin ameliorates neuropathic pain by suppressing HDAC1 expression in the spinal cord of spinal nerve ligation rats. Journal of the Formosan Medical Association, 113(8), 513–520.
Zhang, Z., et al. (2011). Epigenetic suppression of GAD65 expression mediates persistent pain. Nature Medicine, 17(11), 1448–1455.
Kanao-Kanda, M., et al. (2020). Viral vector-mediated gene transfer of glutamic acid decarboxylase for chronic pain treatment: A literature review. Human Gene Therapy, 31(7–8), 405–414.
Dima, R., et al. (2017). Review of literature on low-level laser therapy benefits for nonpharmacological pain control in chronic pain and osteoarthritis. Trials, 5, 6.
Janzadeh, A., et al. (2020). The effect of chondroitinase ABC and photobiomodulation therapy on neuropathic pain after spinal cord injury in adult male rats. Physiology & Behavior, 227, 113141.
Ramezani, F., et al. (2020). Photobiomodulation for spinal cord injury: A systematic review and meta-analysis. Physiology & Behavior, 224, 112977.
Mojarad, N., et al. (2018). The role of low level laser therapy on neuropathic pain relief and interleukin-6 expression following spinal cord injury: an experimental study. Journal of Chemical Neuroanatomy, 87, 60–70.
Neshasteh-Riz, A., et al. (2022). Optimization of the duration and dose of photobiomodulation therapy (660 nm laser) for spinal cord injury in rats. Photobiomodulation, Photomedicine, and Laser Surgery, 40(7), 488–498.
Janzadeh, A., et al. (2016). Photobiomodulation therapy reduces apoptotic factors and increases glutathione levels in a neuropathic pain model. Lasers in Medical Science, 31, 1863–1869.
Azim, K., & Butt, A. M. (2011). GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia, 59(4), 540–553.
Yousefifard, M., et al. (2016). Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model. Stem Cell Research & Therapy, 7(1), 1.
Janzadeh, A., et al. (2017). Combine effect of Chondroitinase ABC and low level laser (660ánm) on spinal cord injury model in adult male rats. Neuropeptides, 65, 90–99.
Hosseini, M., et al. (2020). Simultaneous intrathecal injection of muscimol and endomorphin-1 alleviates neuropathic pain in rat model of spinal cord injury. Brain and Behavior, 10(5), e01576.
Ramezani, F., et al. (2021). Mechanistic aspects of photobiomodulation therapy in the nervous system. Lasers in Medical Science. https://doi.org/10.1007/s10103-021-03277-2
Behroozi, Z., et al. (2023). Evaluation of epigenetic (HDAC, DNMT) and pain (Gad65, TGF) factors following photobiomodulation therapy in a neuropathic pain model. Photochemistry and Photobiology. https://doi.org/10.1111/php.13824
Basso, D. M., Beattie, M. S., & Bresnahan, J. C. (1995). A sensitive and reliable locomotor rating scale for open field testing in rats. Journal of Neurotrauma, 12(1), 1–21.
Chaplan, S. R., et al. (1994). Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods, 53(1), 55–63.
Yoon, C., et al. (1994). Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain, 59(3), 369–376.
Hargreaves, K., et al. (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain, 32(1), 77–88.
Maximow, A. A. (1927). Development of non-granular leucocytes (lymphocytes and monocytes) into polyblasts (macrophages) and fibroblasts in vitro. Proceedings of the Society for Experimental Biology and Medicine, 24(6), 570–572.
Nimmerjahn, A., Kirchhoff, F., & Helmchen, F. (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science, 308(5726), 1314–1318.
Behroozi, Z., et al. (2021). Platelet-rich plasma in umbilical cord blood reduces neuropathic pain in spinal cord injury by altering the expression of ATP receptors. Physiology & Behavior, 228, 113186.
Fischer, A. H., et al. (2008). Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols, 2008(5), p.pdb.prot4986.
Park, J., et al. (2018). Reducing inflammation through delivery of lentivirus encoding for anti-inflammatory cytokines attenuates neuropathic pain after spinal cord injury. Journal of Controlled Release, 290, 88–101.
Madrid, A., et al. (2021). DNA methylation and hydroxymethylation have distinct genome-wide profiles related to axonal regeneration. Epigenetics, 16(1), 64–78.
Saha, R., & Pahan, K. (2006). HATs and HDACs in neurodegeneration: A tale of disconcerted acetylation homeostasis. Cell Death & Differentiation, 13(4), 539–550.
Zamani, A. R. N., et al. (2020). Modulatory effect of photobiomodulation on stem cell epigenetic memory: A highlight on differentiation capacity. Lasers in Medical Science, 35, 299–306.
Martins, M. D., et al. (2021). Photobiomodulation therapy drives massive epigenetic histone modifications, stem cells mobilization and accelerated epithelial healing. Journal of Biophotonics, 14(2), e202000274.
Zaccara, I. M., et al. (2020). Photobiomodulation therapy improves human dental pulp stem cell viability and migration in vitro associated to upregulation of histone acetylation. Lasers in Medical Science, 35, 741–749.
Li, G., Tian, Y., & Zhu, W.-G. (2020). The roles of histone deacetylases and their inhibitors in cancer therapy. Frontiers in Cell and Developmental Biology, 8, 576946.
Wang, G., et al. (2015). HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proceedings of the National Academy of Sciences, 112(9), 2853–2858.
Ouyang, B., et al. (2019). Normalizing HDAC2 levels in the spinal cord alleviates thermal and mechanical hyperalgesia after peripheral nerve injury and promotes GAD65 and KCC2 expression. Frontiers in Neuroscience, 13, 346.
Demyanenko, S., & Uzdensky, A. (2019). Epigenetic alterations induced by photothrombotic stroke in the rat cerebral cortex: Deacetylation of histone H3, upregulation of histone deacetylases and histone acetyltransferases. International Journal of Molecular Sciences, 20(12), 2882.
Jacob, C., et al. (2011). HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nature Neuroscience, 14(4), 429–436.
Gomez-Sanchez, J. A., et al. (2022). Emerging role of hDACs in regeneration and ageing in the peripheral nervous system: Repair schwann cells as pivotal targets. International Journal of Molecular Sciences, 23(6), 2996.
Zhang, S., et al. (2018). Class I histone deacetylase (HDAC) inhibitor CI-994 promotes functional recovery following spinal cord injury. Cell Death & Disease, 9(5), 460.
Lee, J. Y., et al. (2014). Valproic acid protects motor neuron death by inhibiting oxidative stress and endoplasmic reticulum stress-mediated cytochrome C release after spinal cord injury. Journal of Neurotrauma, 31(6), 582–594.
Kim, H. J., et al. (2007). Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. Journal of Pharmacology and Experimental Therapeutics, 321(3), 892–901.
Zhao, J.-Y., et al. (2017). DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nature Communications, 8(1), 14712.
Wang, Y., et al. (2016). Abnormal DNA methylation in the lumbar spinal cord following chronic constriction injury in rats. Neuroscience Letters, 610, 1–5.
Xu, B., et al. (2017). Role of MicroRNA-143 in nerve injury-induced upregulation of Dnmt3a expression in primary sensory neurons. Frontiers in Molecular Neuroscience, 10, 350.
Wu, Z., et al. (2012). Dnmt3a regulates both proliferation and differentiation of mouse neural stem cells. Journal of Neuroscience Research, 90(10), 1883–1891.
Nguyen, S., et al. (2007). Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 236(6), 1663–1676.
Feng, J., et al. (2005). Dynamic expression of de novo DNA methyltransferases Dnmt3a and Dnmt3b in the central nervous system. Journal of Neuroscience Research, 79(6), 734–746.
Nakamura, M., et al. (2003). Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Experimental Neurology, 184(1), 313–325.
Gaudet, A. D., & Fonken, L. K. (2018). Glial cells shape pathology and repair after spinal cord injury. Neurotherapeutics, 15, 554–577.
Setoguchi, T., et al. (2004). Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Experimental Neurology, 189(1), 33–44.
Abematsu, M., et al. (2010). Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. The Journal of Clinical Investigation, 120(9), 3255–3266.
Yiu, G., & He, Z. (2006). Glial inhibition of CNS axon regeneration. Nature Reviews Neuroscience, 7(8), 617–627.
Liddelow, S. A., & Barres, B. A. (2017). Reactive astrocytes: Production, function, and therapeutic potential. Immunity, 46(6), 957–967.
Park, C., et al. (2019). The landscape of myeloid and astrocyte phenotypes in acute multiple sclerosis lesions. Acta Neuropathologica Communications, 7(1), 1–13.
Brenner, M. (2014). Role of GFAP in CNS injuries. Neuroscience Letters, 565, 7–13.
Funding
This study was supported by a scientific project Grant (No: 98-4-68-16528) financed by the Iran University of Medical Science (IUMS) and Islamic Azad University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no competing interests.
Ethical statement
The Institutional Animal Ethical Committee of Iran University of Medical Sciences confirmed all experimental tests and the procedures at present work (IR.IUMS.REC.1399.147).
Consent for publication
All authors consented to publication.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Motamed Nezhad, A., Behroozi, Z., Kookli, K. et al. Evaluation of photobiomodulation therapy (117 and 90s) on pain, regeneration, and epigenetic factors (HDAC 2, DNMT3a) expression following spinal cord injury in a rat model. Photochem Photobiol Sci 22, 2527–2540 (2023). https://doi.org/10.1007/s43630-023-00467-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-023-00467-5