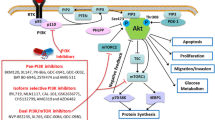
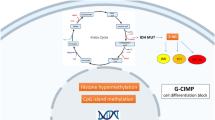
Abstract
Since the discovery of phosphodiesterase-5 (PDE5) enzyme overexpression in the central nervous system (CNS) malignancies, investigations have explored the potential capacity of current PDE5 inhibitor drugs for repositioning in the treatment of brain tumors, notably glioblastoma multiforme (GBM). It has now been recognized that these drugs increase brain tumors permeability and enhance standard chemotherapeutics effectiveness. More importantly, studies have highlighted the promising antitumor functions of PDE5 inhibitors, e.g., triggering apoptosis, suppressing tumor cell growth and invasion, and reversing tumor microenvironment (TME) immunosuppression in the brain. However, contradictory reports have suggested a pro-oncogenic role for neuronal cyclic guanosine monophosphate (cGMP), indicating the beneficial function of PDE5 in the brain of GBM patients. Unfortunately, due to the inconsistent preclinical findings, only a few clinical trials are evaluating the therapeutic value of PDE5 inhibitors in GBM treatment. Accordingly, additional studies should be conducted to shed light on the precise effect of PDE5 inhibitors in GBM biology regarding the existing molecular heterogeneities among individuals. Here, we highlighted and discussed the previously investigated mechanisms underlying the impacts of PDE5 inhibitors in cancers, focusing on GBM to provide an overview of current knowledge necessary for future studies.


Similar content being viewed by others
References
Kavitha A, Chitra L, Kanaga R. Brain tumor segmentation using genetic algorithm with SVM classifier. Int J Adv Res Electr Electron Instrum Eng. 2016;5:1468–71.
Logeswari T, Karnan M. An improved implementation of brain tumor detection using segmentation based on hierarchical self organizing map. Int J Comput Theory Eng. 2010;2:591–5.
Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. The Lancet. 2018;392:432–46.
McFaline-Figueroa JR, Lee EQ. Brain tumors. Am J Med. 2018;131:874–82.
Afshari AR, Mollazadeh H, Mohtashami E, Soltani A, Soukhtanloo M, Hosseini A, et al. Protective role of natural products in glioblastoma multiforme: a focus on nitric oxide pathway. Curr Med Chem. 2021;28:377–400.
Fults D, Pedone CA, Thomas GA, White R. Allelotype of human malignant astrocytoma. Cancer Res. 1990;50:5784–9.
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–25.
Sasaki N, Toyoda M, Ishiwata T. Gangliosides as signaling regulators in cancer. Int J Mol Sci. 2021;22:5076. https://doi.org/10.3390/ijms22105076.
Dong F, Li Q, Xu D, Xiu W, Zeng Q, Zhu X, et al. Differentiation between pilocytic astrocytoma and glioblastoma: a decision tree model using contrast-enhanced magnetic resonance imaging-derived quantitative radiomic features. Eur Radiol. 2019;29:3968–75.
Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol. 2003;30:10–4.
Jalili-Nik M, Sadeghi MM, Mohtashami E, Mollazadeh H, Afshari AR, Sahebkar A. Zerumbone promotes cytotoxicity in human malignant glioblastoma cells through reactive oxygen species (ROS) generation. Oxid Med Cell Longev. 2020. https://doi.org/10.1155/2020/3237983.
Mohtashami E, Shafaei-Bajestani N, Mollazadeh H, Mousavi SH, Jalili-Nik M, Sahebkar A, et al. The current state of potential therapeutic modalities for glioblastoma multiforme: a clinical review. Curr Drug Metab. 2020;21:564–78.
Wu W, Klockow JL, Zhang M, Lafortune F, Chang E, Jin L, et al. Glioblastoma Multiforme (GBM): an overview of current therapies and mechanisms of resistance. Pharmacol Res. 2021. https://doi.org/10.1016/j.phrs.2021.105780.
Afshari AR, Jalili-Nik M, Abbasinezhad-Moud F, Javid H, Karimi M, Mollazadeh H, et al. Antitumor effects of curcuminoids in glioblastoma multiforme: an updated literature review. Curr Med Chem. 2021. https://doi.org/10.2174/0929867327666201111145212.
Maghrouni A, Givari M, Jalili-Nik M, Mollazadeh H, Bibak B, Sadeghi MM, et al. Targeting the PD-1/PD-L1 pathway in glioblastoma multiforme: preclinical evidence and clinical interventions. Int Immunopharmacol. 2021;93: 107403. https://doi.org/10.1016/j.intimp.2021.107403.
Mollazadeh H, Mohtashami E, Mousavi SH, Soukhtanloo M, Vahedi MM, Hosseini A, et al. Deciphering the role of glutamate signaling in glioblastoma multiforme: current therapeutic modalities and future directions. Curr Pharm Des. 2020;26:4777–88.
Afshari AR, Mollazadeh H, Sahebkar A. Minocycline in treating glioblastoma multiforme: far beyond a conventional antibiotic. J Oncol. 2020. https://doi.org/10.1155/2020/8659802.
Barone I, Giordano C, Bonofiglio D, Andò S, Catalano S. Phosphodiesterase type 5 and cancers: progress and challenges. Oncotarget. 2017;8:99179.
Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res. 1996;8:47–52.
Stark S, Sachse R, Liedl T, Hensen J, Rohde G, Wensing G, et al. Vardenafil increases penile rigidity and tumescence in men with erectile dysfunction after a single oral dose. Eur Urol. 2001;40:181–90.
Corbin JD, Beasley A, Blount MA, Francis SH. High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun. 2005;334:930–8.
Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation. 2003;107:3230–5.
Ashour A. Phosphodiesterase-5 inhibitors in the management of cancer. Asian J Biomed Pharm Sci. 2013;3:1–5.
Mostafa T. Oral phosphodiesterase type 5 inhibitors: nonerectogenic beneficial uses. J Sex Med. 2008;5:2502–18.
Catalano S, Panza S, Augimeri G, Giordano C, Malivindi R, Gelsomino L, et al. Phosphodiesterase 5 (PDE5) is highly expressed in cancer-associated fibroblasts and enhances breast tumor progression. Cancers. 2019;11:1740. https://doi.org/10.3390/cancers11111740.
Pantziarka P, Sukhatme V, Crispino S, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)—selective PDE5 inhibitors as anticancer agents. Ecancermedicalscience. 2018. https://doi.org/10.3332/ecancer.2018.824.
Cruz-Burgos M, Losada-Garcia A, Cruz-Hernández CD, Cortés-Ramírez SA, Camacho-Arroyo I, Gonzalez-Covarrubias V, et al. New approaches in oncology for repositioning drugs: the case of PDE5 inhibitor sildenafil. Front Oncol. 2021;11:208. https://doi.org/10.3389/fonc.2021.627229.
Li Q, Shu Y. Pharmacological modulation of cytotoxicity and cellular uptake of anticancer drugs by PDE5 inhibitors in lung cancer cells. Pharm Res. 2014;31:86–96.
Menniti FS, Faraci WS, Schmidt CJ. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discovery. 2006;5:660–70.
Prickaerts J, Van Staveren W, Şik A, Markerink-van Ittersum M, Niewöhner U, Van der Staay F, et al. Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience. 2002;113:351–61.
Santos AI, Carreira BP, Nobre RJ, Carvalho CM, Araújo IM. Stimulation of neural stem cell proliferation by inhibition of phosphodiesterase 5. Stem Cells Int. 2014. https://doi.org/10.1155/2014/878397.
Black KL, Yin D, Ong JM, Hu J, Konda BM, Wang X, et al. PDE5 inhibitors enhance tumor permeability and efficacy of chemotherapy in a rat brain tumor model. Brain Res. 2008;1230:290–302.
Lyday RW, Etters AM, Kim C, Magana F, Pontipiedra GM, Singh NK, et al. PDE5 inhibitors offer novel mechanisms in combination and solo cancer therapy. Curr Cancer Ther Rev. 2017;13:107–19.
Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. https://doi.org/10.3389/fphar.2018.01300.
Petrelli A, Valabrega G. Multitarget drugs: the present and the future of cancer therapy. Expert Opin Pharmacother. 2009;10:589–600.
Chaturvedi VK, Singh A, Singh VK, Singh MP. Cancer nanotechnology: a new revolution for cancer diagnosis and therapy. Curr Drug Metab. 2019;20:416–29.
Shafiee-Nick R, Afshari AR, Mousavi SH, Rafighdoust A, Askari VR, Mollazadeh H, et al. A comprehensive review on the potential therapeutic benefits of phosphodiesterase inhibitors on cardiovascular diseases. Biomed Pharmacother. 2017;94:541–56.
Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–63.
Peak TC, Richman A, Gur S, Yafi FA, Hellstrom WJ. The role of PDE5 inhibitors and the NO/cGMP pathway in cancer. Sex Med Rev. 2016;4:74–84.
Huang W, Sundquist J, Sundquist K, Ji J. Use of phosphodiesterase 5 inhibitors is associated with lower risk of colorectal cancer in men with benign colorectal neoplasms. Gastroenterology. 2019;157:672–81 (e4).
Barone I, Panza S, Giordano C, Gyorffy B, Augimeri G, Gelsomino L, et al. Role of phosphodiesterase 5 (PDE5) in breast cancer stroma: implications for targeted therapy. FASEB J. 2019;33:496. https://doi.org/10.1096/fasebj.2019.33.1_supplement.496.1.
Sponziello M, Verrienti A, Rosignolo F, De Rose RF, Pecce V, Maggisano V, et al. PDE5 expression in human thyroid tumors and effects of PDE5 inhibitors on growth and migration of cancer cells. Endocrine. 2015;50:434–41.
Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520.
Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, et al. Exisulind induction of apoptosis involves guanosine 3′, 5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated β-catenin. Can Res. 2000;60:3338–42.
Li H, Liu L, David ML, Whitehead CM, Chen M, Fetter JR, et al. Pro-apoptotic actions of exisulind and CP461 in SW480 colon tumor cells involve β-catenin and cyclin D1 down-regulation. Biochem Pharmacol. 2002;64:1325–36.
Tinsley HN, Gary BD, Keeton AB, Lu W, Li Y, Piazza GA. Inhibition of PDE5 by sulindac sulfide selectively induces apoptosis and attenuates oncogenic Wnt/β-catenin–mediated transcription in human breast tumor cells. Cancer Prev Res. 2011;4:1275–84.
Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3β∗. J Biol Chem. 2008;283:29572–85.
Sarfati M, Mateo V, Baudet S, Rubio M, Fernandez C, Davi F, et al. Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood J Am Soc Hematol. 2003;101:265–9.
Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89.
Culp MB, Soerjomataram I, Efstathiou JA, Bray F, Jemal A. Recent global patterns in prostate cancer incidence and mortality rates. Eur Urol. 2020;77:38–52.
Hamilton TK, Hu N, Kolomitro K, Bell EN, Maurice DH, Graham CH, et al. Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol. 2013;31:325–30.
Brewer ME, Kim ED. Penile rehabilitation therapy with PDE-V inhibitors following radical prostatectomy: proceed with caution. Adv Urol. 2009. https://doi.org/10.1155/2009/852437.
Aoun F, Slaoui A, Albisinni S, Assenmacher G, de Plaen E, Azzo J-M, et al. Association between phosphodiesterase type 5 inhibitors and prostate cancer: a systematic review. Prog Urol. 2018;28:560–6.
Machen GL, Rajab MH, Pruszynski J, Coffield KS. Phosphodiesterase type 5 inhibitors usage and prostate cancer: a match-paired analysis. Transl Androl Urol. 2017;6:879.
Goluboff ET, Shabsigh A, Saidi JA, Weinstein IB, Mitra N, Heitjan D, et al. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology. 1999;53:440–5.
Goluboff ET, Prager D, Rukstalis D, Giantonio B, Madorsky M, Barken I, et al. Safety and efficacy of exisulind for treatment of recurrent prostate cancer after radical prostatectomy. J Urol. 2001;166:882–6.
Narayanan BA, Reddy BS, Bosland MC, Nargi D, Horton L, Randolph C, et al. Exisulind in combination with celecoxib modulates epidermal growth factor receptor, cyclooxygenase-2, and cyclin D1 against prostate carcinogenesis: in vivo evidence. Clin Cancer Res. 2007;13:5965–73.
Roberts JL, Booth L, Conley A, Cruickshanks N, Malkin M, Kukreja RC, et al. PDE5 inhibitors enhance the lethality of standard of care chemotherapy in pediatric CNS tumor cells. Cancer Biol Ther. 2014;15:758–67.
Das A, Durrant D, Mitchell C, Mayton E, Hoke NN, Salloum FN, et al. Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci. 2010;107:18202–7.
Das A, Durrant D, Mitchell C, Dent P, Batra SK, Kukreja RC. Sildenafil (Viagra) sensitizes prostate cancer cells to doxorubicin-mediated apoptosis through CD95. Oncotarget. 2016;7:4399–413.
Booth L, Roberts JL, Cruickshanks N, Conley A, Durrant DE, Das A, et al. Phosphodiesterase 5 inhibitors enhance chemotherapy killing in gastrointestinal/genitourinary cancer cells. Mol Pharmacol. 2014;85:408–19.
Booth L, Roberts JL, Cruickshanks N, Grant S, Poklepovic A, Dent P. Regulation of osu-03012 toxicity by ER stress proteins and ER stress–inducing drugs. Mol Cancer Ther. 2014;13:2384–98.
Liu N, Mei L, Fan X, Tang C, Ji X, Hu X, et al. Phosphodiesterase 5/protein kinase G signal governs stemness of prostate cancer stem cells through Hippo pathway. Cancer Lett. 2016;378:38–50.
Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79.
Di Cara F, Maile T, Parsons B, Magico A, Basu S, Tapon N, et al. The Hippo pathway promotes cell survival in response to chemical stress. Cell Death Differ. 2015;22:1526–39.
Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505.
Hansen CG, Moroishi T, Guan K-L. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513.
Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–72.
Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57.
Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80.
Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, et al. Tumor-propagating cells and Y ap/T az activity contribute to lung tumor progression and metastasis. EMBO J. 2014;33:468–81.
Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, et al. ERG activates the YAP1 transcriptional program and induces the development of age-related prostate tumors. Cancer Cell. 2015;27:797–808.
Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–38.
Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33.
van Rensburg HJJ, Azad T, Ling M, Hao Y, Snetsinger B, Khanal P, et al. The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. 2018;78:1457–70.
Danley KT, Tan A, Catalona WJ, Leikin R, Helenowski I, Jovanovic B, et al. The association of phosphodiesterase-5 inhibitors with the biochemical recurrence-free and overall survival of patients with prostate cancer following radical prostatectomy. Urol Oncol. 2021. https://doi.org/10.1016/j.urolonc.2021.05.031.
Michl U, Molfenter F, Graefen M, Tennstedt P, Ahyai S, Beyer B, et al. Use of phosphodiesterase type 5 inhibitors may adversely impact biochemical recurrence after radical prostatectomy. J Urol. 2015;193:479–83.
Gallina A, Bianchi M, Gandaglia G, Cucchiara V, Suardi N, Montorsi F, et al. A detailed analysis of the association between postoperative phosphodiesterase type 5 inhibitor use and the risk of biochemical recurrence after radical prostatectomy. Eur Urol. 2015;68:750–3.
Jamnagerwalla J, Howard LE, Vidal AC, Moreira DM, Castro-Santamaria R, Andriole GL, et al. The association between phosphodiesterase type 5 inhibitors and prostate cancer: results from the REDUCE study. J Urol. 2016;196:715–20.
Chavez AH, Coffield KS, Rajab MH, Jo C. Incidence rate of prostate cancer in men treated for erectile dysfunction with phosphodiesterase type 5 inhibitors: retrospective analysis. Asian J Androl. 2013;15:246–8.
Ghahremanloo A, Javid H, Afshari AR, Hashemy SI. Investigation of the role of neurokinin-1 receptor inhibition using aprepitant in the apoptotic cell death through PI3K/Akt/NF-κB signal transduction pathways in colon cancer cells. BioMed Res Int. 2021. https://doi.org/10.1155/2021/1383878.
Rahmani F, Hashemzehi M, Avan A, Barneh F, Asgharzadeh F, Marjaneh RM, et al. Rigosertib elicits potent antitumor responses in colorectal cancer by inhibiting Ras signaling pathway. Cell Signal. 2021;85: 110069. https://doi.org/10.1016/j.cellsig.2021.110069.
Mosier E, Jackson CS, Cox GA, Browning D, Vega KJ. S0295 Regular sildenafil use appears to lower the incidence of colon polyps and colorectal cancer. Official J Am College Gastroenterol. 2020;115:S144. https://doi.org/10.14309/01.ajg.0000703228.18929.1b.
Liu L, Li H, Underwood T, Lloyd M, David M, Sperl G, et al. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharmacol Exp Ther. 2001;299:583–92.
Tinsley HN, Gary BD, Thaiparambil J, Li N, Lu W, Li Y, et al. Colon tumor cell growth–inhibitory activity of sulindac sulfide and other nonsteroidal anti-inflammatory drugs is associated with phosphodiesterase 5 inhibition. Cancer Prev Res. 2010;3:1303–13.
Rice PL, Goldberg RJ, Ray EC, Driggers LJ, Ahnen DJ. Inhibition of extracellular signal-regulated kinase 1/2 phosphorylation and induction of apoptosis by sulindac metabolites. Can Res. 2001;61:1541–7.
Rice PL, Beard KS, Driggers LJ, Ahnen DJ. Inhibition of extracellular-signal regulated kinases 1/2 is required for apoptosis of human colon cancer cells in vitro by sulindac metabolites. Can Res. 2004;64:8148–51.
Aono Y, Horinaka M, Iizumi Y, Watanabe M, Taniguchi T, Yasuda S, et al. Sulindac sulfone inhibits the mTORC1 pathway in colon cancer cells by directly targeting voltage-dependent anion channel 1 and 2. Biochem Biophys Res Commun. 2018;505:1203–10.
Mei X-L, Yang Y, Zhang Y-J, Li Y, Zhao J-M, Qiu J-G, et al. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo. Am J Cancer Res. 2015;5:3311–24.
Li N, Chen X, Zhu B, Ramírez-Alcántara V, Canzoneri JC, Lee K, et al. Suppression of β-catenin/TCF transcriptional activity and colon tumor cell growth by dual inhibition of PDE5 and 10. Oncotarget. 2015;6:27403–15.
Lin F, Hoogendijk L, Buil L, Beijnen JH, van Tellingen O. Sildenafil is not a useful modulator of ABCB1 and ABCG2 mediated drug resistance in vivo. Eur J Cancer. 2013;49:2059–64.
Bhagavathula AS, Tesfaye W, Vidyasagar K. Phosphodiesterase type 5 inhibitors use and risk of colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:2577–84.
Huang W, Sundquist J, Sundquist K, Ji J. Phosphodiesterase-5 inhibitors use and risk for mortality and metastases among male patients with colorectal cancer. Nat Commun. 2020;11:3191. https://doi.org/10.1038/s41467-020-17028-4.
Browning DD. The enduring promise of phosphodiesterase 5 inhibitors for colon cancer prevention. Transl Gastroenterol Hepatol. 2019;4:83. https://doi.org/10.21037/tgh.2019.12.10.
Sutton SS, Magagnoli J, Cummings TH, Hardin JW. The association between phosphodiesterase-5 inhibitors and colorectal cancer in a national cohort of patients. Clin Transl Gastroenterol. 2020;11: e00173. https://doi.org/10.14309/ctg.0000000000000173.
Cea Soriano L, Garcia Rodriguez LA. No association between use of phosphodiesterase 5 inhibitors and colorectal cancer in men with erectile dysfunction. Pharmacoepidemiol Drug Saf. 2020;29:605–8.
Zhang Y, Lo C-H, Giovannucci EL. Phosphodiesterase 5 inhibitor use and risk of conventional and serrated precursors of colorectal cancer. Cancer Epidemiol Prevent Biomarkers. 2021;30:419–21.
Zhu B, Strada SJ. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr Top Med Chem. 2007;7:437–54.
Tinsley HN, Gary BD, Keeton AB, Zhang W, Abadi AH, Reynolds RC, et al. Sulindac sulfide selectively inhibits growth and induces apoptosis of human breast tumor cells by phosphodiesterase 5 inhibition, elevation of cyclic GMP, and activation of protein kinase G. Mol Cancer Ther. 2009;8:3331–40.
Marino N, Collins JW, Shen C, Caplen NJ, Merchant AS, Gökmen-Polar Y, et al. Identification and validation of genes with expression patterns inverse to multiple metastasis suppressor genes in breast cancer cell lines. Clin Exp Metas. 2014;31:771–86.
Catalano S, Campana A, Giordano C, Győrffy B, Tarallo R, Rinaldi A, et al. Expression and function of phosphodiesterase type 5 in human breast cancer cell lines and tissues: implications for targeted therapy. Clin Cancer Res. 2016;22:2271–82.
Pusztai L, Zhen JH, Arun B, Rivera E, Whitehead C, Thompson WJ, et al. Phase I and II study of exisulind in combination with capecitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21:3454–61.
Di X, Gennings C, Bear HD, Graham LJ, Sheth CM, White KL, et al. Influence of the phosphodiesterase-5 inhibitor, sildenafil, on sensitivity to chemotherapy in breast tumor cells. Breast Cancer Res Treat. 2010;124:349–60.
Lin S, Wang J, Wang L, Wen J, Guo Y, Qiao W, et al. Phosphodiesterase-5 inhibition suppresses colonic inflammation-induced tumorigenesis via blocking the recruitment of MDSC. Am J Cancer Res. 2017;7:41–52.
Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. https://doi.org/10.3389/fonc.2013.00049.
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702.
Li W-Q, Qureshi AA, Robinson KC, Han J. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA Intern Med. 2014;174:964–70.
Lian Y, Yin H, Pollak MN, Carrier S, Platt RW, Suissa S, et al. Phosphodiesterase type 5 inhibitors and the risk of melanoma skin cancer. Eur Urol. 2016;70:808–15.
Loeb S, Folkvaljon Y, Lambe M, Robinson D, Garmo H, Ingvar C, et al. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA. 2015;313:2449–55.
Matthews A, Langan SM, Douglas IJ, Smeeth L, Bhaskaran K. Phosphodiesterase type 5 inhibitors and risk of malignant melanoma: matched cohort study using primary care data from the UK clinical practice research datalink. PLoS Med. 2016;13: e1002037. https://doi.org/10.1371/journal.pmed.1002037.
Han X, Han Y, Zheng Y, Sun Q, Ma T, Dai L, et al. Use of phosphodiesterase type 5 inhibitors and risk of melanoma: a meta-analysis of observational studies. Onco Targets Ther. 2018;11:711–20.
Christie A, Vera PL, Higgins M, Kumar S, Lane M, Preston D. Erectile dysfunction medications and skin cancer: an analysis in US veterans. Urology. 2019;126:116–20.
Shkolyar E, Li S, Tang J, Eisenberg ML. Risk of melanoma with phosphodiesterase type 5 inhibitor use among patients with erectile dysfunction, pulmonary hypertension, and lower urinary tract symptoms. J Sex Med. 2018;15:982–9.
Lu YP, Fan S, Liang Z, Song Y, Liu K, Zhou K, et al. Phosphodiesterase type 5 inhibitors and risk of skin cancers in men: a meta-analysis and trial sequential analysis involving 7,479,852 subjects. World J Men’s Health. 2021;39:683–96.
Feng S, Zhou L, Liu Q, He Q, Liao B, Wei X, et al. Are phosphodiesterase type 5 inhibitors associated with increased risk of melanoma?: A systematic review and meta-analysis. Medicine. 2018;97: e9601. https://doi.org/10.1097/MD.0000000000009601.
Hankey W, Sunkel B, Yuan F, He H, Thomas-Ahner JM, Chen Z, et al. Prostate cancer cell phenotypes remain stable following PDE5 inhibition in the clinically relevant range. Transl Nncol. 2020;13: 100797. https://doi.org/10.1016/j.tranon.2020.100797.
Fiscus RR, Johlfs MG. Protein kinase G (PKG): involvement in promoting neural cell survival, proliferation, synaptogenesis, and synaptic plasticity and the use of new ultrasensitive capillary-electrophoresis-based methodologies for measuring PKG expression and molecular actions. Protein Kinase Technol. 2012;68:319–47.
Teich AF, Sakurai M, Patel M, Holman C, Saeed F, Fiorito J, et al. PDE5 exists in human neurons and is a viable therapeutic target for neurologic disease. J Alzheimers Dis. 2016;52:295–302.
Chen JJ, Sun YL, Tiwari AK, Xiao ZJ, Sodani K, Yang DH, et al. PDE 5 inhibitors, sildenafil and vardenafil, reverse multidrug resistance by inhibiting the efflux function of multidrug resistance protein 7 (ATP-binding Cassette C 10) transporter. Cancer Sci. 2012;103:1531–7.
Giordano D, Giorgi M, Sette C, Biagioni S, Augusti-Tocco G. cAMP-dependent induction of PDE5 expression in murine neuroblastoma cell differentiation. FEBS Lett. 1999;446:218–22.
Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–404.
Smith W, McCaslin I, Gokce A, Mandava S, Trost L, Hellstrom W. PDE5 inhibitors: considerations for preference and long-term adherence. Int J Clin Pract. 2013;67:768–80.
García-Barroso C, Ricobaraza A, Pascual-Lucas M, Unceta N, Rico AJ, Goicolea MA, et al. Tadalafil crosses the blood–brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology. 2013;64:114–23.
Liebenberg N, Harvey BH, Brand L, Wegener G, Brink CB. Chronic treatment with the phosphodiesterase type 5 inhibitors sildenafil and tadalafil display anxiolytic effects in Flinders Sensitive Line rats. Metab Brain Dis. 2012;27:337–40.
Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–80.
Booth L, Roberts JL, Tavallai M, Nourbakhsh A, Chuckalovcak J, Carter J, et al. OSU-03012 and viagra treatment inhibits the activity of multiple chaperone proteins and disrupts the blood-brain barrier: implications for anti-cancer therapies. J Cell Physiol. 2015;230:1982–98.
Cesarini V, Martini M, Vitiani LR, Gravina GL, Di Agostino S, Graziani G, et al. Type 5 phosphodiesterase regulates glioblastoma multiforme aggressiveness and clinical outcome. Oncotarget. 2017;8:13223–39.
Hu J, Ljubimova JY, Inoue S, Konda B, Patil R, Ding H, et al. Phosphodiesterase type 5 inhibitors increase Herceptin transport and treatment efficacy in mouse metastatic brain tumor models. PLoS One. 2010;5: e10108. https://doi.org/10.1371/journal.pone.0010108.
Mensch J, Oyarzabal J, Mackie C, Augustijns P. In vivo, in vitro and in silico methods for small molecule transfer across the BBB. J Pharm Sci. 2009;98:4429–68.
Gresser U, Gleiter C. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil-review of the literature. Eur J Med Res. 2002;7:435–46.
Patra JK, Das G, Fraceto LF, Campos EVR, del Pilar R-T, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16:71. https://doi.org/10.1186/s12951-018-0392-8.
Elbardisy B, Galal S, Abdelmonsif DA, Boraie N. Intranasal Tadalafil nanoemulsions: formulation, characterization and pharmacodynamic evaluation. Pharm Dev Technol. 2019;24:1083–94.
Refai H, Hassan D, Abdelmonem R. Development and characterization of polymer-coated liposomes for vaginal delivery of sildenafil citrate. Drug Deliv. 2017;24:278–88.
Zhang P, Zhang Y, Ding X, Xiao C, Chen X. Enhanced nanoparticle accumulation by tumor-acidity-activatable release of sildenafil to induce vasodilation. Biomater Sci. 2020;8:3052–62.
De Rose RF, Cristiano MC, Celano M, Maggisano V, Vero A, Lombardo GE, et al. PDE5 inhibitors-loaded nanovesicles: physico-chemical properties and in vitro antiproliferative activity. Nanomaterials. 2016;6:92. https://doi.org/10.3390/nano6050092.
Ghorbani M, Zarei M, Mahmoodzadeh F, Roshangar L, Nikzad B. Improvement of delivery and anticancer activity of doxorubicin by sildenafil citrate encapsulated with a new redox and pH-responsive nanogel. Int J Polym Mater Polym Biomater. 2020;70:893–902.
Zhang J, Guo J, Zhao X, Chen Z, Wang G, Liu A, et al. Phosphodiesterase-5 inhibitor sildenafil prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in APP/PS1 transgenic mice. Behav Brain Res. 2013;250:230–7.
Titus D, Oliva A, Wilson N, Atkins C. Phosphodiesterase inhibitors as therapeutics for traumatic brain injury. Curr Pharm Des. 2015;21:332–42.
Sugita M, Black KL. Cyclic GMP-specific phosphodiesterase inhibition and intracarotid bradykinin infusion enhances permeability into brain tumors. Cancer Res. 1998;58:914–20.
Shi Z, Tiwari AK, Patel AS, Fu L-W, Chen Z-S. Roles of sildenafil in enhancing drug sensitivity in cancer. Cancer Res. 2011;71:3735–8.
Wu C-P, Lusvarghi S, Tseng P-J, Hsiao S-H, Huang Y-H, Hung T-H, et al. MY-5445, a phosphodiesterase type 5 inhibitor, resensitizes ABCG2-overexpressing multidrug-resistant cancer cells to cytotoxic anticancer drugs. Am J Cancer Res. 2020;10:164–78.
Wang R, Chen W, Zhang Q, Liu Y, Qiao X, Meng K, et al. Phosphodiesterase type 5 inhibitor Tadalafil increases Rituximab treatment efficacy in a mouse brain lymphoma model. J Neurooncol. 2015;122:35–42.
Iratni R, Ayoub MA. Sildenafil in combination therapy against cancer: a literature review. Curr Med Chem. 2021;28:2248–59.
Kashgari FK, Ravna A, Sager G, Lyså R, Enyedy I, Dietrichs ES. Identification and experimental confirmation of novel cGMP efflux inhibitors by virtual ligand screening of vardenafil-analogues. Biomed Pharmacother. 2020;126: 110109. https://doi.org/10.1016/j.biopha.2020.110109.
Kim PK, Zamora R, Petrosko P, Billiar TR. The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol. 2001;1:1421–41.
Soh J-W, Mao Y, Kim M-G, Pamukcu R, Li H, Piazza GA, et al. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6:4136–41.
Tiwari AK, Chen Z-S. Repurposing phosphodiesterase-5 inhibitors as chemoadjuvants. Front Pharmacol. 2013;4:82. https://doi.org/10.3389/fphar.2013.00082.
Lu H, Ning X, Tao X, Ren J, Song X, Tao W, et al. MEKK1 associated with neuronal apoptosis following intracerebral hemorrhage. Neurochem Res. 2016;41:3308–21.
Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci. 1998;95:10541–6.
Aerts I, Van Ostade X, Slegers H. NO-induced activation of cyclic GMP-dependent pathway down regulates ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) protein in rat C6 glioma. Eur J Pharmacol. 2011;659:1–6.
Aerts I, Martin J-J, De Deyn PP, Van Ginniken C, Van Ostade X, Kockx M, et al. The expression of ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (E-NPP1) is correlated with astrocytic tumor grade. Clin Neurol Neurosurg. 2011;113:224–9.
Weed DT, Vella JL, Reis IM, Adriana C, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48.
Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–8.
Weed DT, Zilio S, Reis IM, Sargi Z, Abouyared M, Gomez-Fernandez CR, et al. The reversal of immune exclusion mediated by Tadalafil and an antitumor vaccine also induces PDL1 upregulation in recurrent head and neck squamous cell carcinoma: interim analysis of a phase I clinical trial. Front Immunol. 2019;10:1206. https://doi.org/10.3389/fimmu.2019.01206.
Wirsching H-G, Galanis E, Weller M. Glioblastoma. Handbook Clin Neurol. 2016;134:381–97.
Jalili-Nik M, Afshari AR, Sabri H, Bibak B, Mollazadeh H, Sahebkar A. Zerumbone, a ginger sesquiterpene, inhibits migration, invasion, and metastatic behavior of human malignant glioblastoma multiforme in vitro. BioFactors. 2021. https://doi.org/10.1002/biof.1756.
Alexander BM, Cloughesy TF. Adult glioblastoma. J Clin Oncol. 2017;35:2402–9.
Afshari AR, Motamed-Sanaye A, Sabri H, Soltani A, Karkon-Shayan S, Radvar S, et al. Neurokinin-1 receptor (NK-1R) antagonists: potential targets in the treatment of glioblastoma multiforme. Curr Med Chem. 2021;47:729–39.
Friedmann-Morvinski D. Glioblastoma heterogeneity and cancer cell plasticity. Crit Rev Oncog. 2014;19:327–36.
Jalili-Nik M, Sabri H, Zamiri E, Soukhtanloo M, Roshan MK, Hosseini A, et al. Cytotoxic effects of ferula Latisecta on human glioma U87 cells. Drug Res. 2019;69:665–70.
Afshari AR, Mollazadeh H, Henney NC, Jamialahmad T, Sahebkar A. Effects of statins on brain tumors: a review. Semin Cancer Biol. 2021;73:116–33.
Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20:S2–8.
Ben-Jonathan N, Borcherding DC, Fox S, Hugo ER. Activation of the cGMP/protein kinase G system in breast cancer by the dopamine receptor-1. Cancer Drug Resist. 2019;2:933–47.
Zhu H, Li JT, Zheng F, Martin E, Kots AY, Krumenacker JS, et al. Restoring soluble guanylyl cyclase expression and function blocks the aggressive course of glioma. Mol Pharmacol. 2011;80:1076–84.
Hirsh L, Dantes A, Suh B-S, Yoshida Y, Hosokawa K, Tajima K, et al. Phosphodiesterase inhibitors as anticancer drugs. Biochem Pharmacol. 2004;68:981–8.
Booth L, Roberts JL, Cruickshanks N, Tavallai S, Webb T, Samuel P, et al. PDE5 inhibitors enhance celecoxib killing in multiple tumor types. J Cell Physiol. 2015;230:1115–27.
Piazza GA, Ward A, Chen X, Maxuitenko Y, Coley A, Aboelella NS, et al. PDE5 and PDE10 inhibition activates cGMP/PKG signaling to block Wnt/β-catenin transcription, cancer cell growth, and tumor immunity. Drug Discov Today. 2020;25:1521–7.
Jiang Y, Sheng H, Meng L, Yue H, Li B, Zhang A, et al. RBM5 inhibits tumorigenesis of gliomas through inhibition of Wnt/β-catenin signaling and induction of apoptosis. World J Surg Oncol. 2017;15:9. https://doi.org/10.1186/s12957-016-1084.
Liu X, Wang L, Zhao S, Ji X, Luo Y, Ling F. β-Catenin overexpression in malignant glioma and its role in proliferation and apoptosis in glioblastma cells. Med Oncol. 2011;28:608–14.
Majchrzak-Celińska A, Zielińska-Przyjemska M, Wierzchowski M, Kleszcz R, Studzińska-Sroka E, Kaczmarek M, et al. Methoxy-stilbenes downregulate the transcription of Wnt/β-catenin-dependent genes and lead to cell cycle arrest and apoptosis in human T98G glioblastoma cells. Adv Med Sci. 2021;66:6–20.
Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–81.
Kohsaka S, Hinohara K, Wang L, Nishimura T, Urushido M, Yachi K, et al. Epiregulin enhances tumorigenicity by activating the ERK/MAPK pathway in glioblastoma. Neuro Oncol. 2014;16:960–70.
Han Y, Wu Z, Wu T, Huang Y, Cheng Z, Li X, et al. Tumor-suppressive function of long noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and inactivation of ERK/MAPK signaling. Cell Death Dis. 2016;7: e2123. https://doi.org/10.1038/cddis.2015.407.
Castellino RC, Durden DL. Mechanisms of Disease: the PI3K–Akt–PTEN signaling node—an intercept point for the control of angiogenesis in brain tumors. Nat Clin Pract Neurol. 2007;3:682–93.
Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–92.
Kubiatowski T, Jang T, Lachyankar MB, Salmonsen R, Nabi RR, Quesenberry PJ, et al. Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J Neurosurg. 2001;95:480–8.
Puliyappadamba VT, Hatanpaa KJ, Chakraborty S, Habib AA. The role of NF-κB in the pathogenesis of glioma. Mol Cell Oncol. 2014;1: e963478. https://doi.org/10.4161/23723548.2014.963478.
Korur S, Huber RM, Sivasankaran B, Petrich M, Morin P Jr, Hemmings BA, et al. GSK3β regulates differentiation and growth arrest in glioblastoma. PLoS One. 2009;4: e7443. https://doi.org/10.1371/journal.pone.0007443.
Fianco G, Mongiardi MP, Levi A, De Luca T, Desideri M, Trisciuoglio D, et al. Caspase-8 contributes to angiogenesis and chemotherapy resistance in glioblastoma. Elife. 2017;6: e22593. https://doi.org/10.7554/eLife.22593.
Priya CS, Vidhya R, Kalpana K, Anuradha C. Indirubin-3′-monoxime prevents aberrant activation of GSK-3β/NF-κB and alleviates high fat-high fructose induced Aβ-aggregation, gliosis and apoptosis in mice brain. Int Immunopharmacol. 2019;70:396–407.
Salem MA, Budzyńska B, Kowalczyk J, El Sayed NS, Mansour SM. Tadalafil and bergapten mitigate streptozotocin-induced sporadic Alzheimer’s disease in mice via modulating neuroinflammation, PI3K/Akt, Wnt/β-catenin, AMPK/mTOR signaling pathways. Toxicol Appl Pharmacol. 2021;429: 115697. https://doi.org/10.1016/j.taap.2021.115697.
Nunes AKS, Raposo C, Rocha SWS, de Sousa Barbosa KP, de Almeida Luna RL, da Cruz-Hoefling MA, et al. Involvement of AMPK, IKβα-NFκB and eNOS in the sildenafil anti-inflammatory mechanism in a demyelination model. Brain Res. 2015;1627:119–33.
Walia V, Garg C, Garg M. NO-sGC-cGMP signaling influence the anxiolytic like effect of lithium in mice in light and dark box and elevated plus maze. Brain Res. 2019;1704:114–26.
Pérez-Pérez D, Reyes-Vidal I, Chávez-Cortez EG, Sotelo J, Magaña-Maldonado R. Methylxanthines: potential therapeutic agents for glioblastoma. Pharmaceuticals. 2019;12:130. https://doi.org/10.3390/ph12030130.
Cruz-Burgos M, Losada-Garcia A, Cruz-Hernández CD, Cortés-Ramírez SA, Camacho-Arroyo I, Gonzalez-Covarrubias V, et al. New approaches in oncology for repositioning drugs: the case of PDE5 inhibitor sildenafil. Front Oncol. 2021;11: 627229. https://doi.org/10.3389/fonc.2021.627229.
Xu F, Lv C, Deng Y, Liu Y, Gong Q, Shi J, et al. Icariside II, a PDE5 inhibitor, suppresses oxygen-glucose deprivation/reperfusion-induced primary hippocampal neuronal death through activating the PKG/CREB/BDNF/TrkB signaling pathway. Front Pharmacol. 2020;11:523. https://doi.org/10.3389/fphar.2020.00523.
Yin H, Jiang Y, Zhang Y, Ge H, Yang Z. The inhibition of BDNF/TrkB/PI3K/Akt signal mediated by AG1601 promotes apoptosis in malignant glioma. J Cell Biochem. 2019;120:18771–81.
Charles N, Ozawa T, Squatrito M, Bleau A-M, Brennan CW, Hambardzumyan D, et al. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–52.
Domvri K, Zarogoulidis K, Zogas N, Zarogoulidis P, Petanidis S, Porpodis K, et al. Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J Cancer. 2017;8:3648–56.
Soriano AF, Helfrich B, Chan DC, Heasley LE, Bunn PA, Chou T-C. Synergistic effects of new chemopreventive agents and conventional cytotoxic agents against human lung cancer cell lines. Can Res. 1999;59:6178–84.
Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–88.
Chan DC, Earle KA, Zhao TL, Helfrich B, Zeng C, Baron A, et al. Exisulind in combination with docetaxel inhibits growth and metastasis of human lung cancer and prolongs survival in athymic nude rats with orthotopic lung tumors. Clin Cancer Res. 2002;8:904–12.
Bunn PA Jr, Chan DC, Earle K, Zhao TL, Helfrich B, Kelly K, et al. Preclinical and clinical studies of docetaxel and exisulind in the treatment of human lung cancer. Semin Oncol. 2002;29:87–94.
Muniyan S, Rachagani S, Parte S, Halder S, Seshacharyulu P, Kshirsagar P, et al. Sildenafil potentiates the therapeutic efficacy of docetaxel in advanced prostate cancer by stimulating NO-cGMP signaling. Clin Cancer Res. 2020;26:5720–34.
Booth L, Roberts JL, Poklepovic A, Gordon S, Dent P. PDE5 inhibitors enhance the lethality of pemetrexed through inhibition of multiple chaperone proteins and via the actions of cyclic GMP and nitric oxide. Oncotarget. 2017;8:1449–68.
Booth L, Roberts JL, Poklepovic A, Dent P. PDE5 inhibitors enhance the lethality of [pemetrexed+ sorafenib]. Oncotarget. 2017;8:13464–75.
Booth L, Roberts JL, Poklepovic A, Dent P. [pemetrexed+ sildenafil], via autophagy-dependent HDAC downregulation, enhances the immunotherapy response of NSCLC cells. Cancer Biol Ther. 2017;18:705–14.
Hoang T, Kim K, Merchant J, Traynor AM, McGovern J, Oettel KR, et al. Phase I/II study of gemcitabine and exisulind as second-line therapy in patients with advanced non–small cell lung cancer. J Thorac Oncol. 2006;1:218–25.
Zenitani M, Nojiri T, Uehara S, Miura K, Hosoda H, Kimura T, et al. C-type natriuretic peptide in combination with sildenafil attenuates proliferation of rhabdomyosarcoma cells. Cancer Med. 2016;5:795–805.
Kumazoe M, Tsukamoto S, Lesnick C, Kay NE, Yamada K, Shanafelt TD, et al. Vardenafil, a clinically available phosphodiesterase inhibitor, potentiates the killing effect of EGCG on CLL cells. Br J Haematol. 2015;168:610–3.
Kong D, Jiang Y, Miao X, Wu Z, Liu H, Gong W. Tadalafil enhances the therapeutic efficacy of BET inhibitors in hepatocellular carcinoma through activating hippo pathway. Biochimica et Biophysica Acta (BBA). 2021. https://doi.org/10.1016/j.bbadis.2021.166267.
Luginbuhl A, Johnson J, Harshyne L, Tuluc M, Mardekian S, Leiby B, et al. A window of opportunity trial of preoperative nivolumab with or without tadalafil in squamous cell carcinoma of the head and neck (SCCHN): Safety, clinical, and correlative outcomes. Ann Oncol. 2019;30: v453. https://doi.org/10.1093/annonc/mdz252.008.
Iwasaki T, Onda T, Honda H, Hayashi K, Shibahara T, Nomura T, et al. Over-expression of PDE5 in oral squamous cell carcinoma-effect of sildenafil citrate. Anticancer Res. 2021;41:2297–306.
Ren Y, Zheng J, Yao X, Weng G, Wu L. Essential role of the cGMP/PKG signaling pathway in regulating the proliferation and survival of human renal carcinoma cells. Int J Mol Med. 2014;34:1430–8.
Singh M, Kasna S, Roy S, Aldosary S, Saeedan AS, Ansari MN, et al. Repurposing mechanistic insight of PDE-5 inhibitor in cancer chemoprevention through mitochondrial-oxidative stress intervention and blockade of DuCLOX signalling. BMC Cancer. 2019;19:996. https://doi.org/10.1186/s12885-019-6152-9.
Bimonte VM, Marampon F, Antonioni A, Fittipaldi S, Ferretti E, Pestell RG, et al. Phosphodiesterase type-5 inhibitor tadalafil modulates steroid hormones signaling in a prostate cancer cell line. Int J Mol Sci. 2021;22:754. https://doi.org/10.3390/ijms22020754.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanati, M., Aminyavari, S., Mollazadeh, H. et al. How do phosphodiesterase-5 inhibitors affect cancer? A focus on glioblastoma multiforme. Pharmacol. Rep 74, 323–339 (2022). https://doi.org/10.1007/s43440-021-00349-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00349-6