Abstract
Background
ALS is an incurable neuromuscular degenerative disorder. A familiar form of the disease (fALS) is related to point mutations. The most common one is an expansion of a noncoding GGGGCC hexanucleotide repeat of the C9orf72 gene on chromosome 9p21. An abnormal translation of the C9orf72 gene generates dipeptide repeat proteins that aggregate in the brain. One of the classical approaches for developing treatment against protein aggregation-related diseases is to use chemical chaperones (CSs). In this work, we describe the development of novel 4-phenylbutyric acid (4-PBA) lysosome/ER-targeted derivatives. We assumed that 4-PBA targeting to specific organelles, where protein degradation takes place, might reduce the 4-PBA effective concentration.
Methods
Organic chemistry synthetic methods and solid-phase peptide synthesis (SPPS) were used for preparing the 4-PBA derivatives. The obtained compounds were evaluated in an ALS Drosophila model that expressed C9orf72 repeat expansion, causing eye degeneration. Targeting to lysosome was validated by the 19F-nuclear magnetic resonance (NMR) technique.
Results
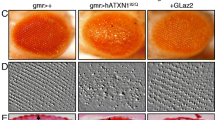
Several synthesized compounds exhibited a significant biological effect by ameliorating the eye degeneration. They blocked the neurodegeneration of fly retina at different efficacy levels. The most active CS was compound 9, which is a peptide derivative and was targeted to ER. Another active compound targeted to lysosome was compound 4.
Conclusions
Novel CSs were more effective than 4-PBA; therefore, they might be used as a new class of drug candidates to treat ALS and other protein misfolding disorders.
Graphic abstract









Similar content being viewed by others
References
van den Bos MAJ, Geevasinga N, Higashihara M, Menon P, Vucic S. Pathophysiology and diagnosis of ALS: insights from advances in neurophysiological techniques. Int J Mol Sci. 2019;20:2818–33.
Oskarsson B, Gendron TF, Staff NP. Amyotrophic lateral sclerosis: an update for 2018. Mayo Clin Proc. 2018;93:1617–28.
Mathis S, Goizet C, Soulages A, Vallat J-M, Le MG. Genetics of amyotrophic lateral sclerosis: a review. J Neurol Sci. 2019;399:217–26.
Rodriguez CM, Todd PK. New pathologic mechanisms in nucleotide repeat expansion disorders. Neurobiol Dis. 2019;130:104515.
Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–44.
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56.
Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68.
Vatsavayai SC, Nana AL, Yokoyama JS, Seeley WW. C9orf72-FTD/ALS pathogenesis: evidence from human neuropathological studies. Acta Neuropathol. 2019;137:1–26.
Zhang Y-J, Jansen-West K, Xu Y-F, Gendron TF, Bieniek KF, Lin W-L, et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014;128:505–24.
Guo Q, Lehmer C, Martínez-Sánchez A, Rudack T, Beck F, Hartmann H, et al. In situ structure of neuronal C9orf72 Poly-GA aggregates reveals proteasome recruitment. Cell. 2018;172:696–705.
Liu Y, Wang J. C9orf72-dependent lysosomal functions regulate epigenetic control of autophagy and lipid metabolism. Autophagy. 2019;15:913–4.
Shao Q, Yang M, Liang C, Ma L, Zhang W, Jiang Z, et al. c9orf72 and smcr8 mutant mice reveal MTORC1 activation due to impaired lysosomal degradation and exocytosis. Autophagy. 2020;16:1635–50.
Corrionero A, Horvitz HR. A C9orf72 ALS/FTD ortholog acts in endolysosomal degradation and lysosomal homeostasis. Curr Biol. 2018;28:1522–35.
Amick J, Ferguson SM. C9orf72: at the intersection of lysosome cell biology and neurodegenerative disease. Traffic. 2017;18:267–76.
Sullivan PM, Zhou X, Robins AM, Paushter DH, Kim D, Smolka MB, et al. The ALS/FTLD associated protein C9orf72 associates with SMCR8 and WDR41 to regulate the autophagy-lysosome pathway. Acta Neuropathol Commun. 2016;4:51.
Yang M, Liang C, Swaminathan K, Herrlinger S, Lai F, Shiekhattar R, et al. A C9ORF72/SMCR8-containing complex regulates ULK1 and plays a dual role in autophagy. Sci Adv. 2016;2:e1601167.
Sellier C, Campanari M-L, Julie Corbier C, Gaucherot A, Kolb-Cheynel I, Oulad-Abdelghani M, et al. Loss of C9ORF72 impairs autophagy and synergizes with polyQ Ataxin-2 to induce motor neuron dysfunction and cell death. EMBO J. 2016;35:1276–97.
Claessen JHL, Kundrat L, Ploegh HL. Protein quality control in the ER: balancing the ubiquitin checkbook. Trends Cell Biol. 2012;22:22–32.
Brodsky JL. Cleaning up: ER-associated degradation to the rescue. Cell. 2012;151:1163–7.
Azoulay-Ginsburg S, Trobiani L, Setini A, Favaloro FL, Giorda E, Jacob A, et al. A lipophilic 4-phenylbutyric acid derivative that prevents aggregation and retention of misfolded proteins. Chem A Eur J. 2020;26:1834–45.
Getter T, Zaks I, Barhum Y, Ben-Zur T, Böselt S, Gregoire S, et al. A chemical chaperone-based drug candidate is effective in a mouse model of amyotrophic lateral sclerosis (ALS). ChemMedChem. 2015;10:850–61.
Cortez L, Sim V. The therapeutic potential of chemical chaperones in protein folding diseases. Prion. 2014;8:197–202.
Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–15.
Tao Y-X, Conn PM. Pharmacoperones as novel therapeutics for diverse protein conformational diseases. Physiol Rev. 2018;98:697–725.
Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 2015;61:45–52.
Gronbeck KR, Rodrigues CMP, Mahmoudi J, Bershad EM, Ling G, Bachour SP, et al. Application of tauroursodeoxycholic acid for treatment of neurological and non-neurological diseases: Is there a potential for treating traumatic brain injury? Neurocrit Care. 2016;25:153–66.
Kusaczuk M, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyric acid: simple structure–multiple effects. Curr Pharm Des. 2015;21:2147–66.
Paganoni S, Macklin EA, Hendrix S, Berry JD, Elliott MA, Maiser S, et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383:919–30.
Hogarth P, Lovrecic L, Krainc D. Sodium phenylbutyrate in Huntington’s disease: a dose-finding study. Mov Disord. 2007;22:1962–4.
Elia AE, Lalli S, Monsurrò MR, Sagnelli A, Taiello AC, Reggiori B, et al. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur J Neurol. 2016;23:45–52.
Ip P, Sharda PR, Cunningham A, Chakrabartty S, Pande V, Chakrabartty A. Quercitrin and quercetin 3-β-d-glucoside as chemical chaperones for the A4V SOD1 ALS-causing mutant. Protein Eng Des Sel. 2017;30:431–40.
Wright GSA, Antonyuk SV, Hasnain SS. A faulty interaction between SOD1 and hCCS in neurodegenerative disease. Sci Rep. 2016;6:27691.
Cudkowicz ME, Andres PL, Macdonald SA, Bedlack RS, Choudry R, Brown RH, et al. Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph Lateral Scler. 2009;10:99–106.
Kaufmann AM, Krise JP. Lysosomal sequestration of amine-containing drugs: analysis and therapeutic implications. J Pharm Sci. 2007;96:729–46.
Andrew CL, Klemm AR, Lloyd JB. Lysosome membrane permeability to amines. Biochim Biophys Acta Biomembr. 1997;1330:71–82.
Bartels AK, Göttert S, Desel C, Schäfer M, Krossa S, Scheidig AJ, et al. KDEL receptor 1 contributes to cell surface association of protein disulfide isomerases. Cell Physiol Biochem. 2019;52:850–68.
Ma WF, Chen HY, Du J, Tan Y, Cai SH. A novel recombinant protein TAT–GFP–KDEL with dual-function of penetrating cell membrane and locating at endoplasm reticulum. J Drug Target. 2009;17:329–33.
Newstead S, Barr F (2020) Molecular basis for KDEL-mediated retrieval of escaped ER-resident proteins—SWEET talking the COPs. J Cell Sci 133:jcs250100
Murshid A, Presley JF. ER-to-Golgi transport and cytoskeletal interactions in animal cells. Cell Mol Life Sci. 2004;61:133–45.
Koon AC, Chan HYE. Drosophila melanogaster as a model organism to study RNA toxicity of repeat expansion-associated neurodegenerative and neuromuscular diseases. Front Cell Neurosci. 2017;11:70.
Gottlieb HE, Kotlyar V, Nudelman A. NMR chemical shifts of common laboratory solvents as trace impurities. J Org Chem. 1997;62:7512–5.
Balalaie S, Mahdidoust M, Eshaghi-Najafabadi R. 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate as an efficient coupling reagent for the amidation and phenylhydrazation of carboxylic acids at room temperature. J Iran Chem Soc. 2007;4:364–9.
Mizielinska S, Grönke S, Niccoli T, Ridler CE, Clayton EL, Devoy A, et al. C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science. 2014;345:1192–4.
Kahremany S, Babaev I, Hasin P, Tamir TY, Ben-Zur T, Cohen G, et al. Computer-aided design and synthesis of 1-{4-[(3,4-dihydroxybenzylidene)amino]phenyl}-5-oxopyrrolidine-3-carboxylic acid as an Nrf2 enhancer. ChemPlusChem. 2018;83:320–33.
Zer Aviv P, Shubely M, Moskovits Y, Viskind O, Albeck A, Vertommen D, et al. A new oxopiperazin-based peptidomimetic molecule inhibits prostatic acid phosphatase secretion and induces prostate cancer cell apoptosis. Chem Select. 2016;1:4658–67.
Xu W, Xu J. C9orf72 dipeptide repeats cause selective neurodegeneration and cell-autonomous excitotoxicity in Drosophila glutamatergic neurons. J Neurosci. 2018;38:7741–52.
Abdullahi A, Stanojcic M, Parousis A, Patsouris D, Jeschke MG. Modeling acute ER stress in vivo and in vitro. Shock. 2017;47:506–13.
Guroff G. PC12 cells as a model of neuronal differentiation. In: Cell culture in the neurosciences. New York: Springer; 1985. p. 245–72.
Bartusik D, Tomanek B. Detection of 19F-labeled biopharmaceuticals in cell cultures with magnetic resonance. Adv Drug Deliv Rev. 2013;65:1056–64.
Sharma S, Schiller MR. The carboxy-terminus, a key regulator of protein function. Crit Rev Biochem Mol Biol. 2019;54:85–102.
Acknowledgements
We acknowledge the Israel Ministry of Science, Technology and Space (MOST), along with the Italian Ministry of Foreign Affairs and the International Cooperation Department, the Directorate General for Country Promotion (the Unit for Scientific and Technological Cooperation) for collaboration grant number 3-13325, which was awarded to A.G. and G.C. The Germany Israel Foundation (GIF) partially supported this work (Grant no. I-1410-201.9/2017) for A.G and S. E. In addition, S. A-G wishes to thank NAAMAT for the Edelson Foundation prize (Grant number: 4) for outstanding women researchers in the field of chemistry and pharmacology and for the Navon fellowship for Ph.D. students (Grant number: 2), awarded by the Israel Ministry of Science, Technology and Space. Last, we thank Steven Manch for the English editing of the article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Azoulay-Ginsburg, S., Di Salvio, M., Weitman, M. et al. Chemical chaperones targeted to the endoplasmic reticulum (ER) and lysosome prevented neurodegeneration in a C9orf72 repeat expansion drosophila amyotrophic lateral sclerosis (ALS) model. Pharmacol. Rep 73, 536–550 (2021). https://doi.org/10.1007/s43440-021-00226-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43440-021-00226-2