Abstract
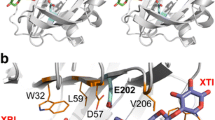
Glucose oxidase (GOD) has many practical applications, but its poor thermostability limits its broader use. In this research, three primary mutants of wild-type GOD were designed using rational mutagenesis, and the GODm mutant was constructed by combinatorial design. The expression, purification, and enzymatic properties of the mutants were studied. The specific enzyme activity of GODm was 2.10-fold higher than that of wild type, and the (kcat/Km) value was increased by 1.45-fold. After treatment at 55 ℃ for 3 h, GODm retained 37.5% of its enzymatic activity, and the half-life (t1/2) of GODm at 55 ℃ and 65 ℃ was 2.28-fold and 3.36-fold higher than that of wild type, respectively. By analyzing the three-dimensional structure of wild type and the GODm mutant, it was found that T30V formed a new hydrogen bond with FAD and strengthened the hydrophobic interaction, D315K optimized the surface electrostatic interaction, and A162T improved the efficiency of the electron pathway. Thus, a novel mutant with improved thermostability and catalytic efficiency was obtained in this research.
Graphic abstract





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Zhang Y, Tsitkov S, Hess H. Proximity does not contribute to activity enhancement in the glucose oxidase-horseradish peroxidase cascade. Nat Commun. 2016;7:13982. https://doi.org/10.1038/ncomms13982.
Pang CP, Yin XX, Zhang GQ, Liu S, Zhou JW, Li JH, Du GC. Current progress and prospects of enzyme technologies in future foods. Syst Microbiol Biomanuf. 2021;1:24–32. https://doi.org/10.1007/s43393-020-00008-6.
Valencia P, Espinoza K, Ramirez C, Franco W, Urtubia A. Technical feasibility of glucose oxidase as a prefermentation treatment for lowering the alcoholic degree of red wine. Am J Enol Vitic. 2017;68:386–9. https://doi.org/10.5344/ajev.2017.16005.
Suroviec AH. Layer-by-layer assembly of glucose oxidase on carbon nanotube modified electrodes. Methods Mol Biol. 2017;1504:203–13. https://doi.org/10.1007/978-1-4939-6499-4_16.
Min SK, Kim DH, Lee J, Ahn HT, Kim MI, Lee J. Self color-changing ordered mesoporous ceria for reagent-free colorimetric biosensing. Nanoscale. 2019;12:1419–24. https://doi.org/10.1039/C9NR09182C.
Wang J, Peiffer M, Hoover K, Rosa C, Zeng R, Felton GW. Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol. 2017;214:1294–306. https://doi.org/10.1111/nph.14429.
Wu S, Li T, Niu H, Zhu Y, Liu Y, Duan Y, Sun Q, Yang X. Effects of glucose oxidase on growth performance, gut function, and cecal microbiota of broiler chickens. Poult Sci. 2018;98:828–41. https://doi.org/10.3382/ps/pey393.
Han XL, Liu GD, Song WX, Qu YB. Production of sodium gluconate from delignified corn cob residue by on-site produced cellulase and co-immobilized glucose oxidase and catalase. Bioresour Technol. 2018;248:248–57. https://doi.org/10.1016/j.biortech.2017.06.109.
Wijma HJ, Floor RJ, Janssen DB. Structure- and sequence-analysis inspired engineering of proteins for enhanced thermostability. Curr Opin Struct Biol. 2013;23:588–94. https://doi.org/10.1016/j.sbi.2013.04.008.
Fakhry B, Asghar KA, Jamshid R. Expression, characterization and one step purification of heterologous glucose oxidase gene from Aspergillus niger ATCC 9029 in Pichia pastoris. EuPA Open Proteom. 2018;19:1–5. https://doi.org/10.1016/j.euprot.2018.09.001.
Muller D. Oxidation von glukose mit extrakten aus Aspergillus niger. Biochem Z. 1928;199:136–70.
Keilin D, Hartree EF. Properties of glucose oxidase (notatin): Addendum. Sedimentation and diffusion of glucose oxidase (notatin). Biochem J. 1948;42:221–9. https://doi.org/10.1042/bj0420221.
Ning X, Zhang Y, Yuan T, Li Q, Tian J, Guan W, Bo L, Wei Z, Xu X, Zhang Y. Enhanced thermostability of glucose oxidase through computer-aided molecular design. Int J Mol Sci. 2018;19:425. https://doi.org/10.3390/ijms19020425.
Siddiqui KS. Defying the activity-stability trade-off in enzymes: taking advantage of entropy to enhance activity and thermostability. Crit Rev Biotechnol. 2016;37:309–22. https://doi.org/10.3109/07388551.2016.1144045.
Vardar G, Altikatoglu M, Basaran Y, Işıldak İ. Synthesis of glucose oxidase-PEG aldehyde conjugates and improvement of enzymatic stability. Artif Cells Nanomed Biotechnol. 2017;46:788–94. https://doi.org/10.1080/21691401.2017.1345920.
Padilla-Martínez S, Martínez-Jothar L, Sampedro JG, Tristan F, Pérez E. Enhanced thermal stability and pH behavior of glucose oxidase on electrostatic interaction with polyethylenimine. Int J Biol Macromol. 2015;75:453–9. https://doi.org/10.1016/j.ijbiomac.2015.02.005.
Bayramoğlu G, Metin AÜ, Altıntas B, Arıca MY. Reversible immobilization of glucose oxidase on polyaniline grafted polyacrylonitrile conductive composite membrane. Bioresour Technol. 2010;101:6881–7. https://doi.org/10.1016/j.biortech.2010.04.025.
Tanatarov D, Huang ZY, Liu YF, Lv XQ, Li JH, Du GC, Liu L. Current advances in design and engineering strategies of industrial enzymes. Syst Microbiol Biomanuf. 2021;1:15–23. https://doi.org/10.1007/s43393-020-00005-9.
Tu T, Wang Y, Huang H, Wang Y, Jiang X, Wang Z, Yao B, Luo H. Improving the thermostability and catalytic efficiency of glucose oxidase from Aspergillus niger by molecular evolution. Food chem. 2019;281:163–70. https://doi.org/10.1016/j.foodchem.2018.12.099.
Ge J, Jiang X, Liu W, Wang Y, Huang H, Bai Y, Su X, Yao B, Luo H. Characterization, stability improvement, and bread baking applications of a novel cold-adapted glucose oxidase from Cladosporium neopsychrotolerans SL16. Food Chem. 2020;310: 125970. https://doi.org/10.1016/j.foodchem.2019.125970.
Vieille C, Zeikus GJ. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev. 2001;65:1–43. https://doi.org/10.1128/MMBR.65.1.1-43.2001.
Hatzinikolaou DG, Macris BJ. Factors regulating production of glucose oxidase by Aspergillus niger. Enzyme Microb Technol. 1995;17:530–4. https://doi.org/10.1016/0141-0229(95)91708-7.
Valdivieso-Ugarte M, Ronchel C, Bauelos O, Velasco J, Ad Rio JL. Expression of an Aspergillus niger glucose oxidase in Saccharomyces cerevisiae and its use to optimize fructo-oligosaccharides synthesis. Biotechnol Prog. 2010;22:1096–101. https://doi.org/10.1021/bp060076k.
Stewart KL, Rathore D, Dodds ED, Cordes MJH. Increased sequence hydrophobicity reduces conformational specificity: a mutational case study of the Arc repressor protein. Proteins. 2019;87:23–33. https://doi.org/10.1002/prot.25613.
Holland JT, Harper JC, Dolan PL, Manginell MM, Arango DC, Rawlings JA, Apblett CA, Brozik SM. Rational redesign of glucose oxidase for improved catalytic function and stability. PLoS ONE. 2012;7: e37924. https://doi.org/10.1371/journal.pone.0037924.
Ostafe R, Prodanovic R, Nazor J, Fischer R. Ultra-high-throughput screening method for the directed evolution of glucose oxidase. Chem Biol. 2014;21:414–21. https://doi.org/10.1016/j.chembiol.2014.01.010.
Mano N. Engineering glucose oxidase for bioelectrochemical applications. Bioelectrochem. 2019;128:218–40. https://doi.org/10.1016/j.bioelechem.2019.04.015.
Noorbatcha IA, Sultan AM, Salleh HM, Amid A. Understanding thermostability factors of Aspergillus niger PhyA Phytase: a molecular dynamics study. Protein J. 2013;32:309–16. https://doi.org/10.1007/s10930-013-9489-y.
Jiang X, Wang YR, Wang Y, Huang HQ, Bai YG, Su XY, Zhang J, Yao B, Tu T, Luo HY. Exploiting the activity-stability trade-off of glucose oxidase from Aspergillus niger using a simple approach to calculate thermostability of mutants. Food Chem. 2021;342: 128270. https://doi.org/10.1016/j.foodchem.2020.128270.
Schweiker KL, Zarrine-Afsar A, Davidson AR, Makhatadze GI. Computational design of the Fyn SH3 domain with increased stability through optimization of surface charge-charge interactions. Protein Sci. 2007;16:2694–702. https://doi.org/10.1110/ps.073091607.
Sedlák E, Sedláková D, Marek J, Hančár J, Žoldák G. Ion-specific protein/water interface determines the Hofmeister effect on the kinetic stability of glucose oxidase. J Phys Chem B. 2019;123:7965–73. https://doi.org/10.1021/acs.jpcb.9b05195.
Leskovac V, Trivic S, Wohlfahrt G, Kandrac J, Pericin D. Glucose oxidase from Aspergillus niger: the mechanism of action with molecular oxygen, quinones, and one-electron acceptors. Int J Biochem cell Biol. 2005;37:731–50. https://doi.org/10.1016/j.biocel.2004.10.014.
Acknowledgements
The authors are grateful for the financial support from the National First-class Discipline Program of Light Industry Technology and Engineering (Grant No. LITE2018-04), the Topnotch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP), and the National Natural Science Foundation of China (NSFC) (32072162).
Author information
Authors and Affiliations
Contributions
HZ: conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, and writing—review and editing. DW: conceptualization, resources, project administration, funding acquisition, and writing—review and editing. PZ: resources and supervision. PC and XY: supervision and validation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, H., Zheng, P., Chen, P. et al. Enhanced thermostability and catalytic efficiency of glucose oxidase in Pichia Pastoris. Syst Microbiol and Biomanuf 2, 296–304 (2022). https://doi.org/10.1007/s43393-021-00057-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-021-00057-5