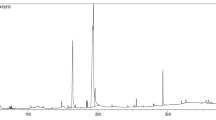
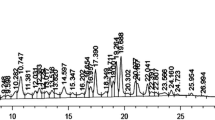
Abstract
Gibberellic acid (GA3) is a natural plant growth regulator that is crucial for plant structural and functional development. We examined the alleviating capacity of brown algae (Dictyota dichotoma) on biochemical and molecular degenerative processes caused by sub-chronic exposure to gibberellic acid resulting in hepatic cell apoptosis. Adult male albino rats were divided into five equal groups: the first group received distilled water, the second group was treated with GA3, the third group was administered D. dichotoma extract suspended in 1% carboxymethylcellulose (CMC), the fourth group was administered both GA3 and D. dichotoma simultaneously, and the fifth group received 1% CMC orally, 5 days per week for a total of 50 days. The results indicated that GA3 induced a significant increase in liver function parameters based on serum levels of alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin, which indicate hepatotoxicity. A marked increase in malondialdehyde (MDA) levels and a marked decrease in reduced glutathione (GSH), glutathione-S-transferase (GST), and superoxide dismutase (SOD) were observed as a result of induction of lipid peroxidation and oxidative stress. Histopathology revealed severely degenerated hepatocytes including cytoplasmic vacuolations and many apoptotic cells with weak Bcl2 expression. Similarly, there was a significant up-regulation of gene and protein expression levels for the pro-apoptotic markers, Caspase-3 and Bax, and an increase in pro-inflammatory marker levels, tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) as well as C-reactive protein (CRP). The co-administration of D. dichotoma restored the disrupted biochemical, histopathological, molecular, and inflammatory changes resulting from GA3 toxicity. Our results confirm the antioxidant, anti-inflammatory, anti-apoptotic, and hepatoprotective potential of D. dichotoma.






Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- ALP:
-
Alkaline phosphatase
- AST:
-
Aspartate aminotransferase
- CMC:
-
Carboxymethylcellulose
- CRP:
-
C-reactive protein
- GSH:
-
Glutathione
- GST:
-
Glutathione-S-transferase
- IL-6:
-
Interleukin-6
- MDA:
-
Malondialdehyde
- qRT-PCR:
-
Quantitative real time-polymerase chain reaction
- SD:
-
Standard deviation
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-alpha
References
Davies PJ (2004) Plant hormones: biosynthesis, signal transduction, action! Springer Science & Business Media, Berlin. https://doi.org/10.1007/978-1-4020-2686-7
Bisht TS, Rawat L, Chakraborty B, Yadav V (2018) A recent advances in use of plant growth regulators (pgrs) in fruit crops-a review. Int J Curr Microbiol Appl Sci 7:1307–1336. https://doi.org/10.20546/ijcmas.2018.705.159
Shi X, Jin F, Huang Y, Du X, Li C, Wang M, Shao H, Jin M, Wang J (2012) Simultaneous determination of five plant growth regulators in fruits by modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction and liquid chromatography–tandem mass spectrometry. J Agric Food Chem 60:60–65. https://doi.org/10.1021/jf204183d
Yue L, Ge C, Feng D, Yu H, Deng H, Fu B (2017) Adsorption–desorption behavior of atrazine on agricultural soils in China. J Environ Sci 57:180–189. https://doi.org/10.1016/j.jes.2016.11.002
Zhang Z, Yang H, Gao Z, Yuan Y, Dong J, Wang Y, Yue T (2017) Identification, synthesis, and safety assessment of thidiazuron [1-Phenyl-3-(1, 2, 3-thidiazol-5-yl) urea] and Its metabolites in kiwifruits. J Agric Food Chem 65:11273–11279. https://doi.org/10.1021/acs.jafc.7b03522
Hafez IH, Osman AR, Sewedan EA, Berber MR (2018) Tailoring of a potential nanoformulated form of gibberellic acid: synthesis, characterization, and field applications on vegetation and flowering. J Agric Food Chem 66:8237–8245. https://doi.org/10.1021/acs.jafc.8b02761
El-Mofty M, Sakr S, Rizk A, Moussa E (1994) Carcinogenic effect of gibberellin A3 in Swiss albino mice. Nutr Cancer 21:183–190. https://doi.org/10.1080/01635589409514316
Tuluce Y, Celik I (2006) Influence of subacute and subchronic treatment of abcisic acid and gibberellic acid on serum marker enzymes and erythrocyte and tissue antioxidant defense systems and lipid peroxidation in rats. Pestic Biochem Physiol 86:85–92. https://doi.org/10.1016/j.pestbp.2006.01.009
Tomlin C (2004) Gibberellic acid. The e-pesticide manual, vol 3, 13th edn. British Crop Protection Council, Hampshire, pp 5–6
Inamadar AC, Palit A (2007) Cutaneous reactions simulating erythema multiforme and Stevens Johnson syndrome due to occupational exposure to a plant-growth regulator. Indian J Dermatol Venereol Leprol 73:330. https://doi.org/10.4103/0378-6323.35734
Zhang L, Sun Y, Xu Z, Zhang W, Huang G, Liu F, Chen L (2021) Insights into pH-dependent transformation of gibberellic acid in aqueous solution: transformation pathway, mechanism and toxicity estimation. J Environ Sci 104:1–10. https://doi.org/10.1016/j.jes.2020.11.009
Hussein MM, Ali HA, Ahmed MM (2015) Ameliorative effects of phycocyanin against gibberellic acid induced hepatotoxicity. Pestic Biochem Physiol 119:28–32. https://doi.org/10.1016/j.pestbp.2015.02.010
Celik I, Tuluce Y, Isik I (2007) Evalution of toxicity of abcisic acid and gibberellic acid in rats: 50 days drinking water study. J Enzyme Inhib Med Chem 22:219–226. https://doi.org/10.1080/14756360600988955
Erin N, Afacan B, Ersoy Y, Ercan F, Balcı MK (2008) Gibberellic acid, a plant growth regulator, increases mast cell recruitment and alters substance P levels. Toxicology 254:75–81. https://doi.org/10.1016/j.tox.2008.09.020
Isik I, Celik I (2015) Investigation of neurotoxic and immunotoxic effects of some plant growth regulators at subacute and subchronic applications on rats. Toxicol Ind Health 31:1095–1105. https://doi.org/10.1177/0748233713487247
Ravikumar S, Srikumar K (2005) Metabolic dysregulation and inhibition of spermatogenesis by gibberellic acid in rat testicular cells. J Environ Biol 26:567–569
Hosseinchi M, Soltanalinejad F, Najafi G, Roshangar L Effect of gibberellic acid on the quality of sperm and in vitro fertilization outcome in adult male rats. In: Veterinary research forum: an international quarterly journal, 2013. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran, p 259
Premalatha R, Jubendradass R, Srikumar K, Mathur P (2014) Gibberellic acid acts as an agonist of steroidogenesis in male rats. Andrologia 46:902–909. https://doi.org/10.1111/and.12171
Hanson JR (2018) Stereochemical aspects of some rearrangements of gibberellic acid. J Chem Res 42:285–290. https://doi.org/10.3184/174751918X15294211268538
Celik I, Turker M, Tuluce Y (2007) Abcisic acid and gibberellic acid cause increased lipid peroxidation and fluctuated antioxidant defense systems of various tissues in rats. J Hazard Mater 148:623–629. https://doi.org/10.1016/j.jhazmat.2007.03.018
Ramajayam G, Sridhar M, Karthikeyan S, Lavanya R, Veni S, Vignesh R, Ilangovan R, Djody SS, Gopalakrishnan V, Arunakaran J (2007) Effects of Aroclor 1254 on femoral bone metabolism in adult male Wistar rats. Toxicology 241:99–105. https://doi.org/10.1016/j.tox.2007.08.086
MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR (2007) Nutritional value of edible seaweeds. Nutr Rev 65:535–543. https://doi.org/10.1301/nr.2007.dec.535-543
Madhusudan C, Manoj S, Rahul K, Rishi CM (2011) Seaweeds: a diet with nutritional, medicinal and industrial value. Res J Med Plant 5:153–157. https://doi.org/10.3923/rjmp.2011.153.157
Antonysamy JMA, Velayutham K, Mani N, Thangaiah S, Irullappan R (2015) Antibacterial, cytotoxic and larvicidal potential of Dictyota bartayresiana Lamour. J Coast Life Med 3:352–355. https://doi.org/10.12980/JCLM.3.2015JCLM-2014-0092
Hwang I-K, Kim H-S, Lee WJ (2005) Polymorphism in the brown alga Dictyota dichotoma (Dictyotales, Phaeophyceae) from Korea. Mar Biol 147:999–1015. https://doi.org/10.1007/s00227-005-1623-8
Coombe DR, Parish CR, Ramshaw IA, Snowden JM (1987) Analysis of the inhibition of tumour metastasis by sulphated polysaccharides. Int J Cancer 39:82–88. https://doi.org/10.1002/ijc.2910390115
Nishino H (1995) Cancer chemoprevention by natural carotenoids and their related compounds. J Cell Biochem 59(S22):231–235. https://doi.org/10.1002/jcb.240590829
Khanavi M, Nabavi M, Sadati N, Shams Ardekani M, Sohrabipour J, Nabavi SMB, Ghaeli P, Ostad SN (2010) Cytotoxic activity of some marine brown algae against cancer cell lines. Biol Res 43:31–37. https://doi.org/10.4067/S0716-97602010000100005
Zhang Z, Teruya K, Yoshida T, Eto H, Shirahata S (2013) Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar Drugs 11(1):81–98. https://doi.org/10.3390/md11010081
Batara DCR (2016) Cytotoxic effect and antioxidant activity of ethanolic extract derived from Dictyota dichotoma (Hudson) JV lamouroux, 1809 Master’s thesis Mariano Marcos State University
Eahamban K, Marimuthu J (2012) Preliminary phytochemical, UV-VIS, HPLC and anti-bacterial studies on Gracilaria corticata J. Ag. Asian Pac J Trop Biomed 2:S568–S574. https://doi.org/10.1016/S2221-1691(12)60275-5
Mahmoud AM, Hussein OE, Ramadan SA (2013) Amelioration of cyclophosphamide-induced hepatotoxicity by the brown seaweed Turbenaria ornata. Int J Clin Toxicol 21:22. https://doi.org/10.14205/2310-4007.2013.01.01.2
Schumann G, Klauke R (2003) New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: preliminary upper reference limits obtained in hospitalized subjects. Clin Chim Acta 327:69–79. https://doi.org/10.1016/S0009-8981(02)00341-8
Doumas BT, Watson WA, Biggs HG (1971) Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta 31:87–96. https://doi.org/10.1016/0009-8981(71)90365-2
Preuss HG, Jarrell ST, Scheckenbach R, Lieberman S, Anderson RA (1998) Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr 17:116–123. https://doi.org/10.1080/07315724.1998.10718736
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Mannervik B, Guthenberg C (1981) [28] Glutathione transferase (human placenta). In: Methods in enzymology, W.B. Jakoby, Editor, vol 77. Elsevier, pp 231–235. https://doi.org/10.1016/S0076-6879(81)77030-7
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474. https://doi.org/10.1111/j.1432-1033.1974.tb03714.x
Huang J, Cui H, Peng X, Fang J, Zuo Z, Deng J, Wu B (2013) The association between splenocyte apoptosis and alterations of Bax, Bcl-2 and caspase-3 mRNA expression, and oxidative stress induced by dietary nickel chloride in broilers. Int J Environ Res Public Health 10:7310–7326. https://doi.org/10.3390/ijerph10127310
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Wei L, Chen Q, Guo A, Fan J, Wang R, Zhang H (2018) Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol 60:1–8. https://doi.org/10.1016/j.intimp.2018.04.016
Suvarna KS, Layton C, Bancroft JD (2019) Bancroft’s theory and practice of histological techniques, 8th edn. Elsevier Health Sciences, Amsterdam. https://doi.org/10.1016/C2015-0-00143-5
Gibson-Corley KN, Olivier AK, Meyerholz DK (2013) Principles for valid histopathologic scoring in research. Vet Pathol 50:1007–1015. https://doi.org/10.1177/0300985813485099
Hsu S-M, Raine L, Fanger H (1981) Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29:577–580. https://doi.org/10.1177/29.4.6166661
Sakr SA, Okdah YA, El-Abd SF (2003) Gibberellin A3 induced histological and histochemical alterations in the liver of albino rats. Sci Asia 29:327–331. https://doi.org/10.2306/scienceasia1513-1874.2003.29.327
Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ (2002) Mechanisms of hepatotoxicity. Toxicol Sci 65:166–176. https://doi.org/10.1093/toxsci/65.2.166
Rodeiro I, Donato M, Martínez I, Hernández I, Garrido G, González-Lavaut J, Menéndez R, Laguna A, Castell J, Gómez-Lechón M (2008) Potential hepatoprotective effects of new Cuban natural products in rat hepatocytes culture. Toxicol In Vitro 22:1242–1249. https://doi.org/10.1016/j.tiv.2008.04.006
Antonisamy JM, Eahamban K (2012) UV–VIS spectroscopic and HPLC studies on Dictyota bartayresiana Lamour. Asian Pac J Trop Biomed 2:S514–S518. https://doi.org/10.1016/S2221-1691(12)60264-0
Hussein WF, Farahat FY, Abass MA, Shehata AS (2011) Hepatotoxic potential of gibberellic acid (GA3) in adult male albino rats. Life Sci J 8:373–383
Alsemeh AE, Moawad RS, Abdelfattah ER (2019) Histological and biochemical changes induced by gibberellic acid in the livers of pregnant albino rats and their offspring: ameliorative effect of Nigella sativa. Anat Sci Int 94:307–323. https://doi.org/10.1007/s12565-019-00488-0
Matanjun P, Mohamed S, Muhammad K, Mustapha NM (2010) Comparison of cardiovascular protective effects of tropical seaweeds, Kappaphycus alvarezii, Caulerpa lentillifera, and Sargassum polycystum, on high-cholesterol/high-fat diet in rats. J Med Food 13:792–800. https://doi.org/10.1089/jmf.2008.1212
Yuan YV, Walsh NA (2006) Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem Toxicol 44:1144–1150. https://doi.org/10.1016/j.fct.2006.02.002
Zhang Q, Li N, Liu X, Zhao Z, Li Z, Xu Z (2004) The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr Res 339:105–111. https://doi.org/10.1016/j.carres.2003.09.015
Parthiban C, Saranya C, Girija K, Hemalatha A, Suresh M, Anantharaman P (2013) Evaluation of in vitro antioxidant properties of some selected seaweeds from Tuticorin coast. Int J Curr Microbiol Appl Sci 2:64–73
Shahidi F (2008) Antioxidants: extraction, identification, application and efficacy measurement. Electron J Environ Agric Food Chem 7:3325–3330
Ibraheem IBM, Abdel-Raouf N, Mohamed HM, Fassihy R, Hamed S (2017) Impact of the microbial suppression by using the brown alga Dictyota dichotoma extract. Egypt J Bot 57(7th International Conf.):205–214
Tariq A, Athar M, Ara J, Sultana V, Ehteshamul-Haque S, Ahmad M (2015) Biochemical evaluation of antioxidant activity and polysaccharides fractions in seaweeds. Glob J Environ Sci Manag 1:47–62. https://doi.org/10.7508/GJESM.2015.01.005
Demirel Z, Yilmaz-Koz FF, Karabay-Yavasoglu UN, Ozdemir G, Sukatar A (2009) Antimicrobial and antioxidant activity of brown algae from the Aegean Sea. J Serb Chem Soc 74:619–628. https://doi.org/10.2298/JSC0906619D
Zubia M, Fabre MS, Kerjean V, Le Lann K, Stiger-Pouvreau V, Fauchon M, Deslandes E (2009) Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem 116:693–701. https://doi.org/10.1016/j.foodchem.2009.03.025
Troudi A, Samet AM, Zeghal N (2010) Hepatotoxicity induced by gibberellic acid in adult rats and their progeny. Exp Toxicol Pathol 62:637–642. https://doi.org/10.1016/j.etp.2009.08.010
El-Shenody RA, Ashour M, Ghobara MME (2019) Evaluating the chemical composition and antioxidant activity of three Egyptian seaweeds: Dictyota dichotoma, Turbinaria decurrens, and Laurencia obtusa. Braz J Food Technol 22:e2018203. https://doi.org/10.1590/1981-6723.20318
Cui J, Placzek WJ (2018) Post-transcriptional regulation of anti-apoptotic BCL2 family members. Int J Mol Sci 19:308. https://doi.org/10.3390/ijms19010308
Xie H, Qin Y-X, Zhou Y-L, Tong L-J, Lin L-P, Geng M-Y, Duan W-H, Ding J (2009) GA3, a new gambogic acid derivative, exhibits potent antitumor activities in vitro via apoptosis-involved mechanisms. Acta Pharmacol Sin 30:346–354. https://doi.org/10.1038/aps.2009.3
Gao Y, Li C, Yin J, Shen J, Wang H, Wu Y, Jin H (2012) Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ Toxicol Pharmacol 33:304–311. https://doi.org/10.1016/j.etap.2011.12.022
Catala A (2007) The ability of melatonin to counteract lipid peroxidation in biological membranes. Curr Mol Med 7:638–649. https://doi.org/10.2174/156652407782564444
Guo Y, Wang W, Chen Y, Sun Y, Li Y, Guan F, Shen Q, Guo Y, Zhang W (2020) Continuous gibberellin A3 exposure from weaning to sexual maturity induces ovarian granulosa cell apoptosis by activating Fas-mediated death receptor signaling pathways and changing methylation patterns on caspase-3 gene promoters. Toxicol Lett 319:175–186. https://doi.org/10.1016/j.toxlet.2019.11.012
Ercolano G, De Cicco P, Ianaro A (2019) New drugs from the sea: Pro-apoptotic activity of sponges and algae derived compounds. Mar Drugs 17:31. https://doi.org/10.3390/md17010031
Bendtzen K (1988) Interleukin 1, interleukin 6 and tumor necrosis factor in infection, inflammation and immunity. Immunol Lett 19:183–192. https://doi.org/10.1016/0165-2478(88)90141-1
Scott D, Kingsley G (2006) Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med 355:704–712. https://doi.org/10.1056/NEJMct055183
Soliman MM, Aldhahrani A, Gaber A, Alsanie WF, Shukry M, Mohamed WA, Metwally MM, Mohamed AA (2021) Impacts of n-acetyl cysteine on gibberellic acid-induced testicular dysfunction through regulation of inflammatory cytokines, steroid and antioxidant activity. Andrologia 53:e14036. https://doi.org/10.1111/and.14036
Kumar A, Takada Y, Boriek AM, Aggarwal BB (2004) Nuclear factor-κB: its role in health and disease. J Mol Med 82:434–448. https://doi.org/10.1007/s00109-004-0555-y
Park JH, Kang S-S, Kim JY, Tchah H (2015) The antioxidant N-acetylcysteine inhibits inflammatory and apoptotic processes in human conjunctival epithelial cells in a high-glucose environment. Investig Ophthalmol Vis Sci 56:5614–5621. https://doi.org/10.1167/iovs.15-16909
Khalaf H, Arafat E, Ghoneim F (2019) A histological, immunohistochemical and biochemical study of the effects of pomegranate peel extracts on gibberellic acid induced oxidative stress in adult rat testes. Biotech Histochem 94:569–582. https://doi.org/10.1080/10520295.2019.1602884
Palanisamy S, Vinosha M, Marudhupandi T, Rajasekar P, Prabhu NM (2017) Isolation of fucoidan from Sargassum polycystum brown algae: structural characterization, in vitro antioxidant and anticancer activity. Int J Biol Macromol 102:405–412. https://doi.org/10.1016/j.ijbiomac.2017.03.182
Yoon W-J, Ham YM, Kim S-S, Yoo B-S, Moon J-Y, Baik JS, Lee NH, Hyun C-G (2009) Suppression of pro-inflammatory cytokines, iNOS, and COX-2 expression by brown algae Sargassum micracanthum in RAW 264.7 macrophages. EurAsian J BioSci 3:130–143. https://doi.org/10.5053/ejobios.2009.3.0.17
Makarenkova I, Akhmatova N, Ermakova S, Besednova N (2017) Morphofunctional changes of dendritic cells induced by sulfated polysaccharides of brown algae. Biochem (Moscow) Suppl Ser B Biomed Chem 11:243–250. https://doi.org/10.18097/PBMC2017630139
Park HY, Han MH, Park C, Jin C-Y, Kim G-Y, Choi I-W, Kim ND, Nam T-J, Kwon TK, Choi YH (2011) Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem Toxicol 49:1745–1752. https://doi.org/10.1016/j.fct.2011.04.020
Acknowledgements
We would like to thank Dr. Doaa Ramadan Ismail, Dr. Ahlam Gamal Khalifa, Dr. Shaimaa Emad Hasssan, and Dr. Ola Gomaa Hussin for their substantial help in the lab work. This work was supported by the Science, Technology and Innovation Funding Authority under Grant number RSY 42932.
Funding
This work was supported by the Science, Technology and Innovation Funding Authority under Grant number RSY 42932.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ali, S., Moselhy, W.A., Mohamed, H.M. et al. Ameliorative effects of Dictyota dichotoma on hepatotoxicity induced by gibberellic acid in albino rats. Toxicol Res. 38, 379–392 (2022). https://doi.org/10.1007/s43188-022-00122-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43188-022-00122-8