Abstract
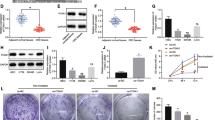
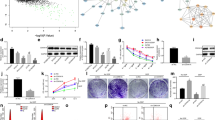
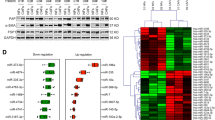
Plenty of pieces of evidence suggest that the resistance to radiotherapy greatly influences the therapeutic effect in cervical cancer (CCa). MicroRNAs (miRNAs) have been reported to regulate cellular processes by acting as tumor suppressors or promoters, thereby driving radioresistance or radiosensitivity. Meanwhile, it has been reported that microRNA-1323 (miR-1323) widely participates in cancer progression and radiotherapy effects. However, the role of miR-1323 is still not clear in CCa. Hence, in this study, we are going to investigate the molecular mechanism of miR-1323 in CCa cells. In the beginning, miR-1323 was found aberrantly upregulated in CCa cells via RT-qPCR assay. Functional assays indicated that miR-1323 was transferred by cancer-associated fibroblasts-secreted (CAFs-secreted) exosomes and miR-1323 downregulation suppressed cell proliferation, migration, invasion, and increased cell radiosensitivity in CCa. Mechanism assays demonstrated that miR-1323 targeted poly(A)-binding protein nuclear 1 (PABPN1). Besides, PABPN1 recruited insulin-like growth factor 2 mRNA binding protein 1 (IGF2BP1) to regulate glycogen synthase kinase 3 beta (GSK-3β) and influenced Wnt/β-catenin signaling pathway. Therefore, rescue experiments were implemented to validate that PABPN1 overexpression rescued the inhibited cancer development and radioresistance induced by the miR-1323 inhibitor. In conclusion, miR-1323 was involved in CCa progression and radioresistance which might provide a novel insight for CCa treatment.
Graphical abstract








Similar content being viewed by others
Data Availability
Research data are not shared.
References
Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, et al. Cervical cancer: screening, diagnosis and staging. Journal of BUON : official journal of the Balkan Union of Oncology. 2016;21(2):320–5.
Petry KU. HPV and cervical cancer. Scandinavian journal of clinical and laboratory investigation Supplementum. 2014;244:59-62; discussion. 2014;10(3109/00365513):936683.
Willmott LJ, Monk BJ. Cervical cancer therapy: current, future and anti-angiogensis targeted treatment. Expert review of anticancer therapy. 2009;9(7):895–903. https://doi.org/10.1586/era.09.58.
Powell ME. Modern radiotherapy and cervical cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2010;20(11 Suppl 2):S49–51. https://doi.org/10.1111/igc.0b013e3181f7b241.
Huang C, Lu H, Li J, Xie X, Fan L, Wang D, et al. SOX2 regulates radioresistance in cervical cancer via the hedgehog signaling pathway. Gynecologic oncology. 2018;151(3):533–41. https://doi.org/10.1016/j.ygyno.2018.10.005.
Li Q, Zhang Y, Jiang Q. SETD3 reduces KLC4 expression to improve the sensitization of cervical cancer cell to radiotherapy. Biochemical and biophysical research communications. 2019;516(3):619–25. https://doi.org/10.1016/j.bbrc.2019.06.058.
Bandyopadhyay A, Mukherjee U, Ghosh S, Ghosh S, Sarkar SK. Pattern of failure with locally advanced cervical cancer– a retrospective audit and analysis of contributory factors. Asian Pacific Journal of Cancer Prevention : APJCP. 2018;19(1):73–9. https://doi.org/10.22034/apjcp.2018.19.1.73.
Sun FD, Wang PC, Luan RL, Zou SH, Du X. MicroRNA-574 enhances doxorubicin resistance through down-regulating SMAD4 in breast cancer cells. European Review for Medical and Pharmacological Sciences. 2018;22(5):1342–50. https://doi.org/10.26355/eurrev_201803_14476.
Nahand JS, Taghizadeh-Boroujeni S, Karimzadeh M, Borran S, Pourhanifeh MH, Moghoofei M, et al. microRNAs: new prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. Journal of cellular physiology. 2019;234(10):17064–99. https://doi.org/10.1002/jcp.28457.
Laengsri V, Kerdpin U, Plabplueng C, Treeratanapiboon L, Nuchnoi P. Cervical cancer markers: epigenetics and microRNAs. Laboratory medicine. 2018;49(2):97–111. https://doi.org/10.1093/labmed/lmx080.
Wang L, Zhao Y, Xiong W, Ye W, Zhao W, Hua Y. MicroRNA-449a Is downregulated in cervical cancer and inhibits proliferation, migration, and invasion. Oncology research and treatment. 2019;42(11):564–71. https://doi.org/10.1159/000502122.
Hua FF, Liu SS, Zhu LH, Wang YH, Liang X, Ma N, et al. MiRNA-338-3p regulates cervical cancer cells proliferation by targeting MACC1 through MAPK signaling pathway. European Review for Medical and Pharmacological Sciences. 2017;21(23):5342–52. https://doi.org/10.26355/eurrev_201712_13919.
Pedroza-Torres A, López-Urrutia E, García-Castillo V, Jacobo-Herrera N, Herrera LA, Peralta-Zaragoza O, et al. MicroRNAs in cervical cancer: evidences for a miRNA profile deregulated by HPV and its impact on radio-resistance. Molecules (Basel, Switzerland). 2014;19(5):6263–81. https://doi.org/10.3390/molecules19056263.
Zhang Y, Li P, Hu J, Zhao LN, Li JP, Ma R, et al. Role and mechanism of miR-4778-3p and its targets NR2C2 and Med19 in cervical cancer radioresistance. Biochemical and biophysical research communications. 2019;508(1):210–6. https://doi.org/10.1016/j.bbrc.2018.11.110.
Wei YQ, Jiao XL, Zhang SY, Xu Y, Li S, Kong BH. MiR-9-5p could promote angiogenesis and radiosensitivity in cervical cancer by targeting SOCS5. European Review for Medical and Pharmacological Sciences. 2019;23(17):7314–26. https://doi.org/10.26355/eurrev_201909_18837.
Zhang F, Yang C, Xing Z, Liu P, Zhang B, Ma X, et al. LncRNA GAS5-mediated miR-1323 promotes tumor progression by targeting TP53INP1 in hepatocellular carcinoma. OncoTargets and therapy. 2019;12:4013–23. https://doi.org/10.2147/ott.S209439.
Zhao H, Zheng C, Wang Y, Hou K, Yang X, Cheng Y, et al. miR-1323 Promotes cell migration in lung adenocarcinoma by targeting Cbl-b and is an early prognostic biomarker. Frontiers in oncology. 2020;10:181. https://doi.org/10.3389/fonc.2020.00181.
Xu Y, Liu M. MicroRNA-1323 downregulation promotes migration and invasion of breast cancer cells by targeting tumour protein D52. Journal of biochemistry. 2020;168(1):83–91. https://doi.org/10.1093/jb/mvaa035.
Li Y, Han W, Ni TT, Lu L, Huang M, Zhang Y, et al. Knockdown of microRNA-1323 restores sensitivity to radiation by suppression of PRKDC activity in radiation-resistant lung cancer cells. Oncology reports. 2015;33(6):2821–8. https://doi.org/10.3892/or.2015.3884.
Qi X, Zhang DH, Wu N, Xiao JH, Wang X, Ma W. ceRNA in cancer: possible functions and clinical implications. Journal of medical genetics. 2015;52(10):710–8. https://doi.org/10.1136/jmedgenet-2015-103334.
Yang F, Ning Z, Ma L, Liu W, Shao C, Shu Y, et al. Exosomal miRNAs and miRNA dysregulation in cancer-associated fibroblasts. Molecular cancer. 2017;16(1):148. https://doi.org/10.1186/s12943-017-0718-4.
Walsh L, Morgia M, Fyles A, Milosevic M. Technological advances in radiotherapy for cervical cancer. Current opinion in oncology. 2011;23(5):512–8. https://doi.org/10.1097/CCO.0b013e3283499d93.
Chu TY, Yang JT, Huang TH, Liu HW. Crosstalk with cancer-associated fibroblasts increases the growth and radiation survival of cervical cancer cells. Radiation research. 2014;181(5):540–7. https://doi.org/10.1667/rr13583.1.
Mei Y, Jiang P, Shen N, Fu S, Zhang J. Identification of miRNA-mRNA regulatory network and construction of prognostic signature in cervical cancer. DNA and cell biology. 2020;39(6):1023–40. https://doi.org/10.1089/dna.2020.5452.
Bahrami A, Hasanzadeh M, ShahidSales S, Yousefi Z, Kadkhodayan S, Farazestanian M, et al. Clinical significance and prognosis value of Wnt signaling pathway in cervical cancer. Journal of cellular biochemistry. 2017;118(10):3028–33. https://doi.org/10.1002/jcb.25992.
Yang Y, Zhou H, Zhang G, Xue X. Targeting the canonical Wnt/β-catenin pathway in cancer radioresistance: updates on the molecular mechanisms. Journal of cancer research and therapeutics. 2019;15(2):272–7. https://doi.org/10.4103/jcrt.JCRT_421_18.
Pereira B, Billaud M, Almeida R. RNA-binding proteins in cancer: old players and new actors. trends in cancer. 2017;3(7):506–28. https://doi.org/10.1016/j.trecan.2017.05.003.
Huang X, Zhang H, Guo X, Zhu Z, Cai H, Kong X. Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) in cancer. Journal of hematology & oncology. 2018;11(1):88. https://doi.org/10.1186/s13045-018-0628-y.
Acknowledgements
Contributions from all participants are appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the ethics committee of Renmin Hospital of Wuhan University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Figure S1
(A) EdU assay detected the proliferation of C33A cells treated with PBS, NFs-exo, CAFs-exo and CAFs-exo+GW4869. (B) Western blot was conducted to examine PABPN1 protein level in CCa cell lines and normal cell line. (C) The protein level of PABPN1 was knocked down in C33A cells. (D) Protein levels of nuclear-β-catenin when PABPN1 was depleted. (E-F) Protein levels of GSK-3β, nuclear-β-catenin, p-β-catenin (S33) and p-β-catenin (S37) after PABPN1 or miR-1323 was inhibited. *P <0.05, **P < 0.01 (PNG 1176 kb)
Rights and permissions
About this article
Cite this article
Fang, F., Guo, C., Zheng, W. et al. Exosome-Mediated Transfer of miR-1323 from Cancer-Associated Fibroblasts Confers Radioresistance of C33A Cells by Targeting PABPN1 and Activating Wnt/β-Catenin Signaling Pathway in Cervical Cancer. Reprod. Sci. 29, 1809–1821 (2022). https://doi.org/10.1007/s43032-021-00820-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00820-y