Abstract
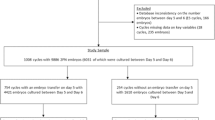
In the current in vitro fertilization and embryo transfer protocol, the 8 blastomeres in the day-3 embryo are selected for transfer because these embryos can produce high rates of blastocyst formation and clinical pregnancy. However, the relationship between the blastomere number in day-2 embryos and the rate of blastocyst formation or clinical pregnancy remains unclear. The purpose of this retrospective study is to explore the relationship between the blastomere number in day-2 embryos and the rate of blastocyst formation or clinical pregnancy. From January 2015 to April 2020, we collected 8126 day-3 embryos (8 blastomeres) from 2282 patients. These embryos were classified into 8 groups (1 blastomere, 2 blastomeres, 3 blastomeres, 4 blastomeres, 5 blastomeres, 6 blastomeres, 7 blastomeres, and 8 blastomeres) based on their blastomeres number on day 2 after insemination. Of these groups, the 4 blastomeres group accounted for the largest proportion (74.44%). The 1 blastomere group accounted for the smallest proportion (0.22%). A total of 3554 day-3 embryos (8 blastomeres) from 1648 patients developed into blastocysts. The rate of blastocyst formation from the 4 blastomeres group was the highest (94.06%). Finally, 800 patients received single day-3 embryos (8 blastomeres) transfer. The rate of clinical pregnancy from 4 blastomeres group was the highest (51.98%). In conclusion, our data provide evidence that the number of blastomeres in day-2 embryos affects the rate of blastocyst formation and clinical pregnancy.

Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Wang S, Ding L, Zhao X, Zhang N, Hu Y, Sun H. Embryo selection for single embryo transfer at day 3 based on combination of cleavage patterns and timing parameters in in vitro fertilization patients. J Reprod Med. 2016;61:254–62.
Awadalla M, Vestal N, McGinnis L, Ahmady A. Effect of age and morphology on live birth rate after cleavage stage embryo transfer. Reprod Sci. 2021;28(1):43–51.
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83.
Deng J, Zhao Q, Cinnioglu C, Kayali R, Lathi RB, Behr B. The impact of culture conditions on blastocyst formation and aneuploidy rates: a comparison between single-step and sequential media in a large academic practice. J Assist Reprod Genet. 2020;37:161–9.
Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15:2394–403.
Puga-Torres T, Blum-Rojas X, Blum-Narváez M. Blastocyst classification systems used in Latin America: is a consensus possible? JBRA Assist Reprod. 2017;21(3):222–9.
Lopez-Regalado ML, Martínez-Granados L, González-Utor A, Ortiz N, Iglesias M, Ardoy M, Castilla JA, Special Interest Group in Quality of ASEBIR (Society for the Study of Reproductive Biology). Critical appraisal of the Vienna consensus: performance indicators for assisted reproductive technology laboratories. Reprod Biomed Online. 2018;37:128–32.
Gardner DK, Balaban B. Assessment of human embryo development using morphological criteria in an era of time-lapse, algorithms and ‘OMICS’: is looking good still important? Mol Hum Reprod. 2016;22:704–18.
Kong X, Yang S, Gong F, Lu C, Zhang S, Lu G, Lin G. The relationship between cell number, division behavior and developmental potential of cleavage stage human embryos: a time-lapse study. PLoS One. 2016;11:e0153697.
Doronin YK, Senechkin IV, Hilkevich LV, Kurcer MA. Cleavage of human embryos: options and diversity. Acta Naturae. 2016;8:88–96.
Shen X, Long H, Gao H, Guo W, Xie Y, Chen D, Cong Y, Wang Y, Li D, Si J, Zhao L, Lyu Q, Kuang Y, Wang L. The valuable reference of live birth rate in the single vitrified-warmed BB/BC/CB blastocyst transfer: the cleavage-stage embryo quality and embryo development speed. Front Physiol. 2020;11:1102.
McCoy RC. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 2017;33:448–63.
Racowsky C, Jackson KV, Cekleniak NA, Fox JH, Hornstein MD, Ginsburg ES. The number of eight-cell embryos is a key determinant for selecting day 3 or day 5 transfer. Fertil Steril. 2000;73:558–64.
Mantzouratou A, Delhanty JD. Aneuploidy in the human cleavage stage embryo. Cytogenet Genome Res. 2011;133(2–4):141–8.
Griffin DK, Ogur C. Chromosomal analysis in IVF: just how useful is it? Reproduction. 2018;156(1):F29–50.
Kim HJ, Park JK, Eum JH, Song H, Lee WS, Lyu SW. Embryo selection based on morphological parameters in a single vitrified-warmed blastocyst transfer cycle. Reprod Sci. 2021;28(4):1060–8.
Luna M, Copperman AB, Duke M, Ezcurra D, Sandler B, Barritt J. Human blastocyst morphological quality is significantly improved in embryos classified as fast on day 3 (>or=10 cells), bringing into question current embryological dogma. Fertil Steril. 2008;89:358–63.
Lee MJ, Lee RK, Lin MH, Hwu YM. Cleavage speed and implantation potential of early-cleavage embryos in IVF or ICSI cycles. J Assist Reprod Genet. 2012;29(8):745–50.
Monk D, Mackay DJG, Eggermann T, Maher ER, Riccio A. Genomic imprinting disorders: lessons on how genome, epigenome and environment interact. Nat Rev Genet. 2019;20(4):235–48.
Herbemont C, Sarandi S, Boujenah J, Cedrin-Durnerin I, Sermondade N, Vivot A, Poncelet C, Grynberg M, Sifer C. Should we consider day-2 and day-3 embryo morphology before day-5 transfer when blastocysts reach a similar good quality? Reprod Biomed Online. 2017;35(5):521–8.
La Salle S. Growing fast or slow: what makes the best embryo? Biol Reprod. 2012;86((5)142):1–2.
Funding
This work was supported by Natural Science Foundation of Hainan Province (No.2019CXTD408, ZDYF2020117), project supported by Hainan Province Clinical Medical Center, and National Natural Science Foundation of China (No. 82072880, 81960283, 81460034).
Author information
Authors and Affiliations
Contributions
Z.M. and Z.L. contributed equally to the manuscript. Y.M. Z.L. and Q.L. conceptualized and designed the study. Z.M, Z.L., Y.Z., J.Z., Y.Y., Y.Z., Y.H. and Q.L. designed and carried out experiments and collected and analyzed data. Z.M., Z.L., Q.L. and Y.M. discussed and edited the paper. All authors contributed to the writing of this manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Approval was obtained from the ethics committee of Hainan Medical University (No. 2015003). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Mi, Z., Liu, Z., Zhang, Y. et al. Number of Blastomeres in Day-2 Embryos Affect the Rates of Blastocyst Formation and Clinical Pregnancy During In Vitro Fertilization Cycles. Reprod. Sci. 28, 3397–3405 (2021). https://doi.org/10.1007/s43032-021-00774-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00774-1