Abstract
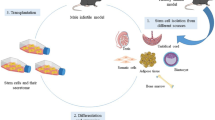
Infertility is a major health problem, and despite improved treatments over the years, there are still some conditions that cannot be treated successfully using a conventional approach. Therefore, new options are being considered and one of them is cell therapy using stem cells. Stem cell treatments for infertility can be divided into two major groups, the first one being direct transplantation of stem cells or their paracrine factors into reproductive organs and the second one being in vitro differentiation into germ cells or gametes. In animal models, all of these approaches were able to improve the reproductive potential of tested animals, although in humans there is still too little evidence to suggest successful use. The reasons for lack of evidence are unavailability of proper material, the complexity of explored biological processes, and ethical considerations. Despite all of the above-mentioned hurdles, researchers were able to show that in women, it seems to be possible to improve some conditions, but in men, no similar clinically important improvement was achieved. To conclude, the data presented in this review suggest that the treatment of infertility with stem cells seems plausible, because some types of treatments have already been tested in humans, achieving live births, while others show great potential only in animal studies, for now.
Similar content being viewed by others
Availability of Data and Material
Not applicable.
References
Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care. Fertil Steril. 2017;108(3):393–406.
Beke A. Genetic causes of female infertility. Exp Suppl. 2019;111:367–83.
Deroux A, Dumestre-Perard C, Dunand-Faure C, Bouillet L, Female HP. Infertility and serum auto-antibodies: a systematic review. Clin Rev Allergy Immunol. 2017;53(1):78–86.
Zhao YX, Chen SR, Su PP, Huang FH, Shi YC, Shi QY, et al. Using mesenchymal stem cells to treat female infertility: an update on female reproductive diseases. Stem Cells Int. 2019;2019:9071720.
Jahanbani Y, Davaran S, Ghahremani-Nasab M, Aghebati-Maleki L, Yousefi M. Scaffold-based tissue engineering approaches in treating infertility. Life Sci. 2020;240:117066.
Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10.
Watt FM, Driskell RR. The therapeutic potential of stem cells. Philos Trans R Soc Lond B Biol Sci. 2010;365(1537):155–63.
Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19(3):193–204.
Lazarini F, Gabellec MM, Moigneu C, de Chaumont F, Olivo-Marin JC, Lledo PM. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J Neurosci. 2014;34(43):14430–42.
Ozakpinar OB, Maurer AM, Ozsavci D. Ovarian stem cells: from basic to clinical applications. World J Stem Cells. 2015;7(4):757–68.
Bao R, Xu P, Wang Y, Wang J, Xiao L, Li G, et al. Bone marrow derived mesenchymal stem cells transplantation rescues premature ovarian insufficiency induced by chemotherapy. Gynecol Endocrinol. 2018;34(4):320–6.
Song D, Zhong Y, Qian C, Zou Q, Ou J, Shi Y, et al. Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model. Biomed Res Int. 2016;2016:2517514.
Liu R, Zhang X, Fan Z, Wang Y, Yao G, Wan X, et al. Human amniotic mesenchymal stem cells improve the follicular microenvironment to recover ovarian function in premature ovarian failure mice. Stem Cell Res Ther. 2019;10(1):299.
Herraiz S, Pellicer N, Romeu M, Pellicer A. Treatment potential of bone marrow-derived stem cells in women with diminished ovarian reserves and premature ovarian failure. Curr Opin Obstet Gynecol. 2019;31(3):156–62.
Yang Z, Du X, Wang C, Zhang J, Liu C, Li Y, et al. Therapeutic effects of human umbilical cord mesenchymal stem cell-derived microvesicles on premature ovarian insufficiency in mice. Stem Cell Res Ther. 2019;10(1):250.
Li J, Mao Q, He J, She H, Zhang Z, Yin C. Human umbilical cord mesenchymal stem cells improve the reserve function of perimenopausal ovary via a paracrine mechanism. Stem Cell Res Ther. 2017;8(1):55.
Shen J, Cao D, Sun JL. Ability of human umbilical cord mesenchymal stem cells to repair chemotherapy-induced premature ovarian failure. World J Stem Cells. 2020;12(4):277–87.
Zhang X, Zhang L, Li Y, Yin Z, Feng Y, Ji Y. Human umbilical cord mesenchymal stem cells (hUCMSCs) promotes the recovery of ovarian function in a rat model of premature ovarian failure (POF). Gynecol Endocrinol. 2021:1–5. https://doi.org/10.1080/09513590.2021.1878133 Epub ahead of print.
Zheng Q, Fu X, Jiang J, Zhang N, Zou L, Wang W, et al. Umbilical cord mesenchymal stem cell transplantation prevents chemotherapy-induced ovarian failure via the NGF/TrkA pathway in rats. Biomed Res Int. 2019;2019:6539294.
Cui L, Bao H, Liu Z, Man X, Liu H, Hou Y, et al. hUMSCs regulate the differentiation of ovarian stromal cells via TGF-β1/Smad3 signaling pathway to inhibit ovarian fibrosis to repair ovarian function in POI rats. Stem Cell Res Ther. 2020;11(1):386.
Wang Z, Wei Q, Wang H, Han L, Dai H, Qian X, et al. Mesenchymal stem cell therapy using human umbilical cord in a rat model of autoimmune-induced premature ovarian failure. Stem Cells Int. 2020;2020:3249495.
Sun L, Li D, Song K, Wei J, Yao S, Li Z, et al. Exosomes derived from human umbilical cord mesenchymal stem cells protect against cisplatin-induced ovarian granulosa cell stress and apoptosis in vitro. Sci Rep. 2017;7(1):2552.
Hong L, Yan L, Xin Z, Hao J, Liu W, Wang S, et al. Protective effects of human umbilical cord mesenchymal stem cell-derived conditioned medium on ovarian damage. J Mol Cell Biol. 2020;12(5):372–85.
Zhang Q, Bu S, Sun J, Xu M, Yao X, He K, et al. Paracrine effects of human amniotic epithelial cells protect against chemotherapy-induced ovarian damage. Stem Cell Res Ther. 2017;8(1):270.
Ding C, Zou Q, Wang F, Wu H, Chen R, Lv J, et al. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res Ther. 2018;9(1):55.
Ling L, Feng X, Wei T, Wang Y, Wang Y, Wang Z, et al. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 2019;10(1):46.
Feng X, Ling L, Zhang W, Liu X, Wang Y, Luo Y, et al. Effects of human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation in situ on primary ovarian insufficiency in SD rats. Reprod Sci. 2020;27(7):1502–12.
Cho J, Kim TH, Seok J, Jun JH, Park H, Kweon M, et al. Vascular remodeling by placenta-derived mesenchymal stem cells restores ovarian function in ovariectomized rat model via the VEGF pathway. Lab Invest. 2021;101(3):304–17.
Seok J, Park H, Choi JH, Lim JY, Kim KG, Kim GJ. Placenta-derived mesenchymal stem cells restore the ovary function in an ovariectomized rat model via an antioxidant effect. Antioxidants (Basel). 2020;9(7):591.
Li H, Zhao W, Wang L, Luo Q, Yin N, Lu X, et al. Human placenta-derived mesenchymal stem cells inhibit apoptosis of granulosa cells induced by IRE1α pathway in autoimmune POF mice. Cell Biol Int. 2019;43(8):899–909.
Kim KH, Kim EY, Kim GJ, Ko JJ, Cha KY, Koong MK, et al. Human placenta-derived mesenchymal stem cells stimulate ovarian function via miR-145 and bone morphogenetic protein signaling in aged rats. Stem Cell Res Ther. 2020;11(1):472.
Choi JH, Seok J, Lim SM, Kim TH, Kim GJ. Microenvironmental changes induced by placenta-derived mesenchymal stem cells restore ovarian function in ovariectomized rats via activation of the PI3K-FOXO3 pathway. Stem Cell Res Ther. 2020;11(1):486.
Ding C, Zou Q, Wang F, Wu H, Wang W, Li H, et al. HGF and BFGF secretion by human adipose-derived stem cells improves ovarian function during natural aging via activation of the SIRT1/FOXO1 signaling pathway. Cell Physiol Biochem. 2018;45(4):1316–32.
Terraciano P, Garcez T, Ayres L, Durli I, Baggio M, Kuhl CP, et al. Cell therapy for chemically induced ovarian failure in mice. Stem Cells Int. 2014;2014:720753.
Sun M, Wang S, Li Y, Yu L, Gu F, Wang C, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80.
Takehara Y, Yabuuchi A, Ezoe K, Kuroda T, Yamadera R, Sano C, et al. The restorative effects of adipose-derived mesenchymal stem cells on damaged ovarian function. Lab Invest. 2013;93(2):181–93.
Su J, Ding L, Cheng J, Yang J, Li X, Yan G, et al. Transplantation of adipose-derived stem cells combined with collagen scaffolds restores ovarian function in a rat model of premature ovarian insufficiency. Hum Reprod. 2016;31(5):1075–86.
Shojafar E, Soleimani Mehranjani M, Shariatzadeh SMA. Adipose derived mesenchymal stem cells improve the structure and function of autografted mice ovaries through reducing oxidative stress and inflammation: a stereological and biochemical analysis. Tissue Cell. 2019;56:23–30.
Manavella DD, Cacciottola L, Payen VL, Amorim CA, Donnez J, Dolmans MM. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. Mol Hum Reprod. 2019;25(4):184–93.
Mehdinia Z, Ashrafi M, Fathi R, Taheri P, Valojerdi MR. Restoration of estrous cycles by co-transplantation of mouse ovarian tissue with MSCs. Cell Tissue Res. 2020;381(3):509–25.
Gabr H, Rateb MA, El Sissy MH, Ahmed Seddiek H, Ali Abdelhameed Gouda S. The effect of bone marrow-derived mesenchymal stem cells on chemotherapy induced ovarian failure in albino rats. Microsc Res Tech. 2016;79(10):938–47.
Badawy A, Sobh MA, Ahdy M, Abdelhafez MS. Bone marrow mesenchymal stem cell repair of cyclophosphamide-induced ovarian insufficiency in a mouse model. Int J Womens Health. 2017;9:441–7.
Mohamed SA, Shalaby SM, Abdelaziz M, Brakta S, Hill WD, Ismail N, et al. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci. 2018;25(1):51–63.
Wang Z, Yang T, Liu S, Chen Y. Effects of bone marrow mesenchymal stem cells on ovarian and testicular function in aging Sprague-Dawley rats induced by D-galactose. Cell Cycle. 2020;20:1–11.
Grady ST, Watts AE, Thompson JA, Penedo MCT, Konganti K, Hinrichs K. Effect of intra-ovarian injection of mesenchymal stem cells in aged mares. J Assist Reprod Genet. 2019;36(3):543–56.
Zarbakhsh S, Safari R, Sameni HR, Yousefi B, Safari M, Khanmohammadi N, et al. Effects of co-administration of bone marrow stromal cells and L-carnitine on the recovery of damaged ovaries by performing chemotherapy model in rat. Int J Fertil Steril. 2019;13(3):196–202.
Sameni HR, Seiri M, Safari M, Tabrizi Amjad MH, Khanmohammadi N, Zarbakhsh S. Bone marrow stromal cells with the granulocyte colony-stimulating factor in the management of chemotherapy-induced ovarian failure in a rat model. Iran J Med Sci. 2019;44(2):135–45.
Volkova N, Yukhta M, Goltsev A. Mesenchymal stem cells in restoration of fertility at experimental pelvic inflammatory disease. Stem Cells Int. 2017;2017:2014132.
Kalhori Z, Azadbakht M, Soleimani Mehranjani M, Shariatzadeh MA. Improvement of the folliculogenesis by transplantation of bone marrow mesenchymal stromal cells in mice with induced polycystic ovary syndrome. Cytotherapy. 2018;20(12):1445–58.
Edessy M, Hosni HN, Shady Y, Waf Y, Bakr S, Kamel M. Autologous stem cells therapy, the first baby of idiopathic premature ovarian failure. Acta Med Int. 2016;3:19–23.
Gupta S, Lodha P, Karthick MS, Tandulwadkar SR. Role of autologous bone marrow-derived stem cell therapy for follicular recruitment in premature ovarian insufficiency: review of literature and a case report of world’s first baby with ovarian autologous stem cell therapy in a perimenopausal woman of age 45 year. J Hum Reprod Sci. 2018;11(2):125–30.
Igboeli P, El Andaloussi A, Sheikh U, Takala H, ElSharoud A, McHugh A, et al. Intraovarian injection of autologous human mesenchymal stem cells increases estrogen production and reduces menopausal symptoms in women with premature ovarian failure: two case reports and a review of the literature. J Med Case Rep. 2020;14(1):108.
Herraiz S, Romeu M, Buigues A, Martínez S, Díaz-García C, Gómez-Seguí I, et al. Autologous stem cell ovarian transplantation to increase reproductive potential in patients who are poor responders. Fertil Steril. 2018;110(3):496–505.
Shin DM, Liu R, Klich I, Wu W, Ratajczak J, Kucia M, et al. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24(8):1450–61.
Magnúsdóttir E, Surani MA. How to make a primordial germ cell. Development. 2014;141(2):245–52.
Hackett JA, Zylicz JJ, Surani MA. Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 2012;28(4):164–74.
Morohaku K, Tanimoto R, Sasaki K, Kawahara-Miki R, Kono T, Hayashi K, et al. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc Natl Acad Sci U S A. 2016;113(32):9021–6.
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–5.
Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303.
Ohta H, Kurimoto K, Okamoto I, Nakamura T, Yabuta Y, Miyauchi H, et al. In vitro expansion of mouse primordial germ cell-like cells recapitulates an epigenetic blank slate. EMBO J. 2017;36(13):1888–907.
Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, et al. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160(1-2):253–68.
Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. 2015;17(2):178–94.
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–21.
Zhang H, Panula S, Petropoulos S, Edsgärd D, Busayavalasa K, Liu L, et al. Adult human and mouse ovaries lack DDX4-expressing functional oogonial stem cells. Nat Med. 2015;21(10):1116–8.
Silvestris E, Cafforio P, D’Oronzo S, Felici C, Silvestris F, Loverro G. In vitro differentiation of human oocyte-like cells from oogonial stem cells: single-cell isolation and molecular characterization. Hum Reprod. 2018;33(3):464–73.
Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Rülicke T, et al. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009;18(1):137–49.
Esmaeilian Y, Atalay A, Erdemli E. Putative germline and pluripotent stem cells in adult mouse ovary and their in vitro differentiation potential into oocyte-like and somatic cells. Zygote. 2017;25(3):358–75.
Bhartiya D, Sharma D. Ovary does harbor stem cells - size of the cells matter! J Ovarian Res. 2020;13(1):39.
Bharti D, Jang SJ, Lee SY, Lee SL, Rho GJ. In vitro generation of oocyte like cells and their in vivo efficacy: how far we have been succeeded. Cells. 2020;9(3):557.
Zuo W, Xie B, Li C, Yan Y, Zhang Y, Liu W, et al. The clinical applications of endometrial mesenchymal stem cells. Biopreserv Biobank. 2018;16(2):158–64 29265881.
Cervelló I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6(6):e21221.
Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–45.
Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–51.
Liu Y, Zhang Z, Yang F, Wang H, Liang S, Wang H, et al. The role of endometrial stem cells in the pathogenesis of endometriosis and their application to its early diagnosis†. Biol Reprod. 2020;102(6):1153–9.
Simoni M, Taylor HS. Therapeutic strategies involving uterine stem cells in reproductive medicine. Curr Opin Obstet Gynecol. 2018;30(3):209–16.
Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod. 2010;82(6):1076–87.
Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman’s syndrome. J Hum Reprod Sci. 2011;4(1):43–8.
Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman’s syndrome: a novel cell based therapy. J Hum Reprod Sci. 2014;7(2):93–8.
Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96.
Cao Y, Sun H, Zhu H, Zhu X, Tang X, Yan G, et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: a phase I clinical trial. Stem Cell Res Ther. 2018;9(1):192.
Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–9.
Sudoma I, Pylyp L, Kremenska Y, Goncharova Y. Application of autologous adipose-derived stem cells for thin endometrium treatment in patients with failed ART programs. J Stem Cell Ther Transplant. 2019;3:001–8.
Lee SY, Shin JE, Kwon H, Choi DH, Kim JH. Effect of autologous adipose-derived stromal vascular fraction transplantation on endometrial regeneration in patients of Asherman’s syndrome: a pilot study. Reprod Sci. 2020;27(2):561–8.
Jazedje T, Perin PM, Czeresnia CE, Maluf M, Halpern S, Secco M, et al. Human fallopian tube: a new source of multipotent adult mesenchymal stem cells discarded in surgical procedures. J Transl Med. 2009;7:46.
Snegovskikh V, Mutlu L, Massasa E, Taylor HS. Identification of putative fallopian tube stem cells. Reprod Sci. 2014;21(12):1460–4.
Chang YH, Chu TY, Ding DC. Human fallopian tube epithelial cells exhibit stemness features, self-renewal capacity, and Wnt-related organoid formation. J Biomed Sci. 2020;27(1):32.
Zhu M, Iwano T, Takeda S. Fallopian tube basal stem cells reproducing the epithelial sheets in vitro-stem cell of fallopian epithelium. Biomolecules. 2020;10(9):1270.
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, et al. The notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989.
Yucer N, Holzapfel M, Jenkins Vogel T, Lenaeus L, Ornelas L, Laury A, et al. Directed differentiation of human induced pluripotent stem cells into fallopian tube epithelium. Sci Rep. 2017;7(1):10741.
Li Z, Zhang Z, Chen X, Zhou J, Xiao XM. Treatment evaluation of Wharton’s jelly-derived mesenchymal stem cells using a chronic salpingitis model: an animal experiment. Stem Cell Res Ther. 2017;8(1):232.
Liao W, Tang X, Li X, Li T. Therapeutic effect of human umbilical cord mesenchymal stem cells on tubal factor infertility using a chronic salpingitis murine model. Arch Gynecol Obstet. 2019;300(2):421–9.
Li Z, Zhang Z, Ming WK, Chen X, Xiao XM. Tracing GFP-labeled WJMSCs in vivo using a chronic salpingitis model: an animal experiment. Stem Cell Res Ther. 2017;8(1):272.
Almasry SM, Elfayomy AK, El-Sherbiny MH. Regeneration of the fallopian tube mucosa using bone marrow mesenchymal stem cell transplantation after induced chemical injury in a rat model. Reprod Sci. 2018;25(5):773–81.
Labarta E, de Los Santos MJ, Escribá MJ, Pellicer A, Herraiz S. Mitochondria as a tool for oocyte rejuvenation. Fertil Steril. 2019;111(2):219–26.
Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16(3):513–6.
Cohen J, Scott R, Alikani M, Schimmel T, Munné S, Levron J, et al. Ooplasmic transfer in mature human oocytes. Mol Hum Reprod. 1998;4(3):269–80.
Lanzendorf SE, Mayer JF, Toner J, Oehninger S, Saffan DS, Muasher S. Pregnancy following transfer of ooplasm from cryopreserved-thawed donor oocytes into recipient oocytes. Fertil Steril. 1999;71(3):575–7.
Woods DC, Tilly JL. Autologous germline mitochondrial energy transfer (AUGMENT) in human assisted reproduction. Semin Reprod Med. 2015;33(6):410–21.
Labarta E, de Los Santos MJ, Herraiz S, Escribá MJ, Marzal A, Buigues A, Pellicer A (2019) Autologous mitochondrial transfer as a complementary technique to intracytoplasmic sperm injection to improve embryo quality in patients undergoing in vitro fertilization-a randomized pilot study. Fertil Steril 111(1):86-96
Fakih MH, El Shmoury M, Szeptycki J, dela Cruz DB, Lux C, Verjee S, et al. The AUGMENTSM treatment: physician reported outcomes of the initial global patient experience. JFIV Reprod Med Genet. 2015;3:154.
Oktay K, Baltaci V, Sonmezer M, Turan V, Unsal E, Baltaci A, et al. Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod Sci. 2015;22(12):1612–7.
Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol. 1980;124(2):211–5.
Cocuzza M, Alvarenga C, Pagani R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 68 Suppl. 2013;1(Suppl 1):15–26.
Mehmood S, Aldaweesh S, Junejo NN, Altaweel WM, Kattan SA, Alhathal N. Microdissection testicular sperm extraction: overall results and impact of preoperative testosterone level on sperm retrieval rate in patients with nonobstructive azoospermia. Urol Ann. 2019;11(3):287–93.
Taitson PF, Filho MA, Radaelli MRM. Testicular sperm extraction in men with Sertoli cell-only testicular histology - 1680 cases. JBRA Assist Reprod. 2019;23(3):246–9.
von Eckardstein S, Simoni M, Bergmann M, Weinbauer GF, Gassner P, Schepers AG, et al. Serum inhibin B in combination with serum follicle-stimulating hormone (FSH)is a more sensitive marker than serum FSH alone for impaired spermatogenesis in men, but cannot predict the presence of sperm in testicular tissue samples. J Clin Endocrinol Metab. 1999;84(7):2496–501.
Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: a unique subset of men with nonobstructive azoospermia. J Urol. 2007;178(2):608–12.
Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91(24):11303–7.
Jafarian A, Lakpour N, Sadeghi MR, Salehkhou S, Akhondi MM. Transplantation of spermatogonial stem cells suspension into rete testis of azoospermia mouse model. Urol J. 2018;15(1):40–7.
Kanatsu-Shinohara M, Ogonuki N, Matoba S, Ogura A, Shinohara T. Autologous transplantation of spermatogonial stem cells restores fertility in congenitally infertile mice. Proc Natl Acad Sci U S A. 2020;117(14):7837–44.
Nagano M, Patrizio P, Brinster RL. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil Steril. 2002;78(6):1225–33.
Mulder CL, Catsburg LAE, Zheng Y, de Winter-Korver CM, van Daalen SKM, van Wely M, et al. Long-term health in recipients of transplanted in vitro propagated spermatogonial stem cells. Hum Reprod. 2018;33(1):81–90.
Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, et al. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69(2):612–6.
Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, et al. Propagation of human spermatogonial stem cells in vitro. JAMA. 2009;302(19):2127–34.
Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reprod Biol Endocrinol. 2011;9:141.
Dong L, Gul M, Hildorf S, Pors SE, Kristensen SG, Hoffmann ER, et al. Xeno-free propagation of spermatogonial stem cells from infant boys. Int J Mol Sci. 2019;20(21):5390.
Goharbakhsh L, Mohazzab A, Salehkhou S, Heidari M, Zarnani AH, Parivar K, et al. Isolation and culture of human spermatogonial stem cells derived from testis biopsy. Avicenna J Med Biotechnol. 2013;5(1):54–61.
Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, et al. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102(2):566-580.e7.
Cai Y, Wang J, Zou K. The progresses of spermatogonial stem cells sorting using fluorescence-activated cell sorting. Stem Cell Rev Rep. 2020;16(1):94–102.
Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305(23):2416–8.
Shetty G, Mitchell JM, Meyer JM, Wu Z, Lam TNA, Phan TT, et al. Restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation. Andrology https://doi. 2020;8:1428–41. https://doi.org/10.1111/andr.12807.
Forbes CM, Flannigan R, Schlegel PN. Spermatogonial stem cell transplantation and male infertility: current status and future directions. Arab J Urol. 2017;16(1):171–80.
Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471(7339):504–7.
Oblette A, Rives N, Dumont L, Rives A, Verhaeghe F, Jumeau F, et al. Assessment of sperm nuclear quality after in vitro maturation of fresh or frozen/thawed mouse pre-pubertal testes. Mol Hum Reprod. 2017;23(10):674–84.
Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian J Androl. 2012;14(2):285–93.
Huleihel M, Nourashrafeddin S, Plant TM. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J Androl. 2015;17(6):972–80.
Ishikura Y, Yabuta Y, Ohta H, Hayashi K, Nakamura T, Okamoto I, et al. In vitro derivation and propagation of spermatogonial stem cell activity from mouse pluripotent stem cells. Cell Rep. 2016;17(10):2789–804.
de Michele F, Poels J, Weerens L, Petit C, Evrard Z, Ambroise J, et al. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum Reprod. 2016;32(1):32–45.
Sun M, Yuan Q, Niu M, Wang H, Wen L, Yao C, et al. Efficient generation of functional haploid spermatids from human germline stem cells by three-dimensional-induced system. Cell Death Differ. 2018;25(4):749–66.
Cremades N, Bernabeu R, Barros A, Sousa M. In-vitro maturation of round spermatids using co-culture on Vero cells. Hum Reprod. 1999;14(5):1287–93.
Tanaka A, Nagayoshi M, Awata S, Tanaka I, Kusunoki H. Differentiation of human round spermatids into motile spermatozoa through in vitro coculture with Vero cells. Reprod Med Biol. 2009;8(4):169–75.
Kadam P, Ntemou E, Onofre J, Van Saen D, Goossens E. Does co-transplantation of mesenchymal and spermatogonial stem cells improve reproductive efficiency and safety in mice? Stem Cell Res Ther. 2019;10(1):310.
Karimaghai N, Tamadon A, Rahmanifar F, Mehrabani D, Raayat Jahromi A, Zare S, et al. Spermatogenesis after transplantation of adipose tissue-derived mesenchymal stem cells in busulfan-induced azoospermic hamster. Iran J Basic Med Sci. 2018;21(7):660–7.
Li X, Xu A, Li K, Zhang J, Li Q, Zhao G, et al. CXCR4-SF1 bifunctional adipose-derived stem cells benefit for the treatment of Leydig cell dysfunction-related diseases. J Cell Mol Med. 2020;24(8):4633–45.
Curley M, Gonzalez ZN, Milne L, Hadoke P, Handel I, Péault B, et al. Human adipose-derived pericytes display steroidogenic lineage potential in vitro and influence Leydig cell regeneration in vivo in rats. Sci Rep. 2019;9(1):15037.
Badawy AA, El-Magd MA, AlSadrah SA, Alruwaili MM. Altered expression of some miRNAs and their target genes following mesenchymal stem cell treatment in busulfan-induced azoospermic rats. Gene. 2020;737:14448.
Abdelaziz MH, Salah El-Din EY, El-Dakdoky MH, Ahmed TA. The impact of mesenchymal stem cells on doxorubicin-induced testicular toxicity and progeny outcome of male prepubertal rats. Birth Defects Res. 2019;111(13):906–19.
Meligy FY, Abo Elgheed AT, Alghareeb SM. Therapeutic effect of adipose-derived mesenchymal stem cells on Cisplatin induced testicular damage in adult male albino rat. Ultrastruct Pathol. 2019;43(1):28–55.
Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, et al. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: the sperm generation. Biomed Res Int. 2013;2013:529589.
Author information
Authors and Affiliations
Contributions
Petric, P., and Stimpfel, M.: Writing, editing, and data collection
Vrtacnik-Bokal, E.: Writing and editing
Corresponding author
Ethics declarations
Ethics Approval
This is a review; therefore, no ethical consents were needed.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Petric, P., Vrtacnik-Bokal, E. & Stimpfel, M. Is It Possible to Treat Infertility with Stem Cells?. Reprod. Sci. 28, 1733–1745 (2021). https://doi.org/10.1007/s43032-021-00566-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00566-7