Abstract
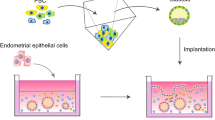
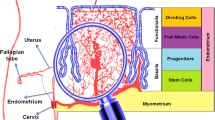
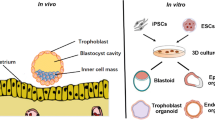
The endometrium is one of the most dynamic organs in the human body. Until now, cell lines have furthered the understanding of endometrial biology and associated diseases, but they failed to recapitulate the key physiological aspects of the endometrium, especially as it relates to its complex architecture and functions. Organoid culture systems have become an alternative approach to reproduce biological functions of tissues in vitro. Endometrial organoids have now been established from stem/progenitor cells and/or differentiated cells by several methods, which represents a promising tool to gain a deeper understanding of this dynamic organ. In this review, we will discuss the establishment, characteristics, applications, and potential challenges and directions of endometrial organoids.

Similar content being viewed by others
Availability of Data and Material (Data Transparency)
Not applicable.
Code Availability (Software Application or Custom Code)
Not applicable.
References
Ferenczy A, Bergeron C. Histology of the human endometrium: from birth to senescence. Ann N Y Acad Sci. 1991;622:6–27.
David L, Keefe KPW. Chapter 2 – Reproductive Physiology. General Gynecology. 2007:21–41.
Hawkins SM, Matzuk MM. The menstrual cycle: basic biology. Ann N Y Acad Sci. 2008;1135:10–8.
Ruiz-Alonso M, Blesa D, Simón C. The genomics of the human endometrium. Biochim Biophys Acta. 2012;1822(12):1931–42.
Evans J, Salamonsen LA, Winship A, Menkhorst E, Nie G, Gargett CE, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654–67.
Ulukus M, Cakmak H, Arici A. The role of endometrium in endometriosis. J Soc Gynecol Investig. 2006;13(7):467–76.
Senturk LM, Erel CT. Thin endometrium in assisted reproductive technology. Curr Opin Obstet Gynecol. 2008;20(3):221–8.
Benagiano G, Brosens I. The endometrium in adenomyosis. Womens Health (Lond). 2012;8(3):301–12.
Kyo S, Sato S, Nakayama K. Cancer-associated mutations in normal human endometrium: Surprise or expected? Cancer Sci. 2020.
Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin Reprod Med. 2013;31(2):109–24.
Stouffer RL, Woodruff TK. nonhuman primates: a vital model for basic and applied research on female reproduction, prenatal development, and women’s health. ILAR J. 2017;58(2):281–94.
Lim HJ, Wang H. Uterine disorders and pregnancy complications: insights from mouse models. J Clin Invest. 2010;120(4):1004–15.
Ochoa-Bernal MA, Fazleabas AT. Physiologic Events of Embryo Implantation and Decidualization in Human and Non-Human Primates. Int J Mol Sci. 2020;21(6).
Song Y, Joshi NR, Vegter E, Hrbek S, Lessey BA, Fazleabas AT. Establishment of an immortalized endometriotic stromal cell line from human ovarian endometrioma. Reprod Sci. 2020.
Zuberi A, Lutz C. Mouse models for drug discovery. Can new tools and technology improve translational power? ILAR J. 2016;57(2):178–85.
Fellmann C, Gowen BG, Lin PC, Doudna JA, Corn JE. Cornerstones of CRISPR-Cas in drug discovery and therapy. Nat Rev Drug Discov. 2017;16(2):89–100.
Matsumoto H. Molecular and cellular events during blastocyst implantation in the receptive uterus: clues from mouse models. J Reprod Dev. 2017;63(5):445–54.
Wu SP, Emery OM, DeMayo FJ. Molecular studies on pregnancy with mouse models. Curr Opin Physiol. 2020;13:123–7.
Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28(1):27–35.
Filant J, Spencer TE. Endometrial glands are essential for blastocyst implantation and decidualization in the mouse uterus. Biol Reprod. 2013;88(4):93.
Vasquez YM, Wang X, Wetendorf M, Franco HL, Mo Q, Wang T, et al. FOXO1 regulates uterine epithelial integrity and progesterone receptor expression critical for embryo implantation. PLoS Genet. 2018;14(11):e1007787.
Wang X, Li X, Wang T, Wu SP, Jeong JW, Kim TH, et al. SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of Ihh. Nat Commun. 2018;9(1):4421.
Finn CA, Martin L. Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol Reprod. 1972;7(1):82–6.
Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod. 1998;59(1):160–8.
Strakova Z, Reed J, Ihnatovych I. Human transcriptional coactivator with PDZ-binding motif (TAZ) is downregulated during decidualization. Biol Reprod. 2010;82(6):1112–8.
Strug MR, Su RW, Kim TH, Jeong JW, Fazleabas A. The notch family transcription factor, RBPJκ, modulates glucose transporter and ovarian steroid hormone receptor expression during decidualization. Reprod Sci. 2019;26(6):774–84.
Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi. 1985;37(7):1103–11.
Nishida M. The Ishikawa cells from birth to the present. Hum Cell. 2002;15(3):104–17.
Zeitvogel A, Baumann R, Starzinski-Powitz A. Identification of an invasive, N-cadherin-expressing epithelial cell type in endometriosis using a new cell culture model. Am J Pathol. 2001;159(5):1839–52.
Schutgens F, Verhaar MC, Rookmaaker MB. Pluripotent stem cell-derived kidney organoids: an in vivo-like in vitro technology. Eur J Pharmacol. 2016;790:12–20.
Leibel SL, McVicar RN, Winquist AM, Niles WD, Snyder EY. Generation of complete multi-cell type lung organoids from human embryonic and patient-specific induced pluripotent stem cells for infectious disease modeling and therapeutics validation. Curr Protoc Stem Cell Biol. 2020;54(1):e118.
Naumovska E, Aalderink G, Wong Valencia C, Kosim K, Nicolas A, Brown S, et al. Direct on-chip differentiation of intestinal tubules from induced pluripotent stem cells. Int J Mol Sci. 2020;21(14).
Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68(12):2228–37.
Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144(10):1775–86.
Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–77.
Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, et al. Trophoblast organoids as a model for maternal-fetal interactions during human placentation. Nature. 2018;564(7735):263–7.
Deane JA, Cousins FL, Gargett CE. Endometrial organoids: in vitro models for endometrial research and personalized medicine. Biol Reprod. 2017;97(6):781–3.
Breslin S, O'Driscoll L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncotarget. 2016;7(29):45745–56.
Hwang TJ, Carpenter D, Lauffenburger JC, Wang B, Franklin JM, Kesselheim AS. Failure of investigational drugs in late-stage clinical development and publication of trial results. JAMA Intern Med. 2016;176(12):1826–33.
Li M, Izpisua Belmonte JC. Organoids - preclinical models of human disease. N Engl J Med. 2019;380(6):569–79.
Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020:1–14.
Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus. 2020;10(2):20190079.
Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, et al. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2020;105(3).
Seishima R, Leung C, Yada S, Murad KBA, Tan LT, Hajamohideen A, et al. Neonatal Wnt-dependent Lgr5 positive stem cells are essential for uterine gland development. Nat Commun. 2019;10(1):5378.
Syed SM, Kumar M, Ghosh A, Tomasetig F, Ali A, Whan RM, et al. Endometrial Axin2(+) cells drive epithelial homeostasis, regeneration, and cancer following oncogenic transformation. Cell Stem Cell. 2020;26(1):64–80.e13.
Kirk D, Alvarez RB. Morphologically stable epithelial vesicles cultured from normal human endometrium in defined media. In Vitro Cell Dev Biol. 1986;22(10):604–14.
Rinehart CA Jr, Lyn-Cook BD, Kaufman DG. Gland formation from human endometrial epithelial cells in vitro. In Vitro Cell Dev Biol. 1988;24(10):1037–41.
Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, et al. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports. 2018;11(2):537–51.
Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil. 1994;101(2):327–32.
Blauer M, Heinonen PK, Martikainen PM, Tomas E, Ylikomi T. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum Reprod. 2005;20(4):864–71.
Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, Spencer TE. Self-renewing endometrial epithelial organoids of the human uterus. Proc Natl Acad Sci U S A. 2019;116(46):23132–42.
Haider S, Gamperl M, Burkard TR, Kunihs V, Kaindl U, Junttila S, et al. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology. 2019;160(10):2282–97.
Hjollund NH, Jensen TK, Bonde JP, Henriksen TB, Andersson AM, Kolstad HA, et al. Spontaneous abortion and physical strain around implantation: a follow-up study of first-pregnancy planners. Epidemiology. 2000;11(1):18–23.
Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94.
Guzeloglu-Kayisli O, Basar M, Arici A. Basic aspects of implantation. Reprod BioMed Online. 2007;15(6):728–39.
Luddi A, Pavone V, Semplici B, Governini L, Criscuoli M, Paccagnini E, et al. Organoids of human endometrium: a powerful in vitro model for the endometrium-embryo cross-talk at the implantation site. Cells. 2020;9(5).
Hennes A, Held K, Boretto M, De Clercq K, Van den Eynde C, Vanhie A, et al. Functional expression of the mechanosensitive PIEZO1 channel in primary endometrial epithelial cells and endometrial organoids. Sci Rep. 2019;9(1):1779.
Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS One. 2014;9(3):e90913.
van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478).
Simon C, Greening DW, Bolumar D, Balaguer N, Salamonsen LA, Vilella F. Extracellular vesicles in human reproduction in health and disease. Endocr Rev. 2018;39(3):292–332.
Kurian NK, Modi D. Extracellular vesicle mediated embryo-endometrial cross talk during implantation and in pregnancy. J Assist Reprod Genet. 2019;36(2):189–98.
Luddi A, Zarovni N, Maltinti E, Governini L, Leo V, Cappelli V, et al. Clues to non-invasive implantation window monitoring: isolation and characterisation of endometrial exosomes. Cells. 2019;8(8).
Villasante A, Marturano-Kruik A, Ambati SR, Liu Z, Godier-Furnemont A, Parsa H, et al. Recapitulating the size and cargo of tumor exosomes in a tissue-engineered model. Theranostics. 2016;6(8):1119–30.
Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9(1):13012.
Ke X, Yan R, Sun Z, Cheng Y, Meltzer A, Lu N, et al. Esophageal adenocarcinoma-derived extracellular vesicle MicroRNAs induce a neoplastic phenotype in gastric organoids. Neoplasia. 2017;19(11):941–9.
Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–98.
Practice committee of the american society for reproductive medicine. Treatment of pelvic pain associated with endometriosis. Fertil Steril 2008;90(5 Suppl):S260-S269.
Endometriosis and infertility: a committee opinion. Fertil Steril. 2012;98(3):591-8.
Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–51.
Yang HL, Zhou WJ, Chang KK, Mei J, Huang LQ, Wang MY, et al. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction. 2017;154(6):815–25.
Wendel JRH, Wang X, Smith LJ, Hawkins SM. Three-dimensional biofabrication models of endometriosis and the endometriotic microenvironment. Biomedicines. 2020;8(11).
Braun MM, Overbeek-Wager EA, Grumbo RJ. Diagnosis and Management of Endometrial Cancer. Am Fam Physician. 2016;93(6):468–74.
Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. 2017;29(1):47–58.
Van Nyen T, Moiola CP, Colas E, Annibali D, Amant F. Modeling endometrial cancer: past, present, and future. Int J Mol Sci. 2018;19(8).
Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057.
Li X, Feng Y, Lin JF, Billig H, Shao R. Endometrial progesterone resistance and PCOS. J Biomed Sci. 2014;21(1):2.
Li Y, Tang P, Cai S, Peng J, Hua G. Organoid based personalized medicine: from bench to bedside. Cell Regen. 2020;9(1):21.
Adli M. The CRISPR tool kit for genome editing and beyond. Nat Commun. 2018;9(1):1911.
Ringel T, Frey N, Ringnalda F, Janjuha S, Cherkaoui S, Butz S, et al. Genome-Scale CRISPR Screening in Human Intestinal Organoids Identifies Drivers of TGF-β Resistance. Cell Stem Cell. 2020;26(3):431–40.e8.
Acknowledgments
This study was funded by NIH Grants R01 HD042280 and HD083273 to A.T.F.
Funding
This research was supported by NIH grants RO1 HD042280 and HD083273 to A.T.F.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declared no potential conflicts of interest.
Ethics Approval (Include Appropriate Approvals or Waivers)
Not applicable.
Consent to Participate (Include Appropriate Statements)
Not applicable.
Consent for Publication (Include Appropriate Statements)
The authors declared that the work has not been published in whole or in part elsewhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Song, Y., Fazleabas, A.T. Endometrial Organoids: A Rising Star for Research on Endometrial Development and Associated Diseases. Reprod. Sci. 28, 1626–1636 (2021). https://doi.org/10.1007/s43032-021-00471-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00471-z