Abstract
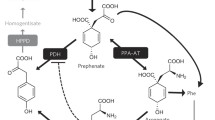
The tyrosine metabolism pathway serves as a starting point for the production of a variety of structurally diverse natural compounds in plants, such as tocopherols, plastoquinone, ubiquinone, betalains, salidroside, benzylisoquinoline alkaloids, and so on. Among these, tyrosine-derived metabolites, tocopherols, plastoquinone, and ubiquinone are essential to plant survival. In addition, this pathway provides us essential micronutrients (e.g., vitamin E and ubiquinone) and medicine (e.g., morphine, salidroside, and salvianolic acid B). However, our knowledge of the plant tyrosine metabolism pathway remains rudimentary, and genes encoding the pathway enzymes have not been fully defined. In this review, we summarize and discuss recent advances in the tyrosine metabolism pathway, key enzymes, and important tyrosine-derived metabolites in plants.


Similar content being viewed by others
References
Amesz J (1973) The function of plastoquinone in photosynthetic electron transport. Biochim Biophys Acta 301:35–51
Araji S, Grammer TA, Gertzen R, Anderson SD, Mikulic-Petkovsek M, Veberic R, Phu ML, Solar A, Leslie CA, Dandekar AM, Escobar MA (2014) Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol 164:1191–1203
Bak S, Kahn RA, Nielsen HL, Moller BL, Halkier BA (1998) Cloning of three A-type cytochromes P450, CYP71E1, CYP98, and CYP99 from Sorghum bicolor (L.) Moench by a PCR approach and identification by expression in Escherichia coli of CYP71E1 as a multifunctional cytochrome P450 in the biosynthesis of the cyanogenic glucoside dhurrin. Plant Mol Biol 36:393–405
Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA (2016) Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat Plants. 2:16050
Beaudegnies R, Edmunds AJ, Fraser TE, Hall RG, Hawkes TR, Mitchell G, Schaetzer J, Wendeborn S, Wibley J (2009) Herbicidal 4-hydroxyphenylpyruvate dioxygenase inhibitors—a review of the triketone chemistry story from a Syngenta perspective. Bioorg Med Chem 17:4134–4152
Beaudoin-Eagan LD, Thorpe TA (1985) Tyrosine and phenylalanine ammonia lyase activities during shoot initiation in tobacco callus cultures. Plant Physiol 78:438–441
Berner M, Krug D, Bihlmaier C, Vente A, Muller R, Bechthold A (2006) Genes and enzymes involved in caffeic acid biosynthesis in the actinomycete Saccharothrix espanaensis. J Bacteriol 188:2666–2673
Block A, Widhalm JR, Fatihi A, Cahoon RE, Wamboldt Y, Elowsky C, Mackenzie SA, Cahoon EB, Chapple C, Dudareva N, Basset GJ (2014) The origin and biosynthesis of the benzenoid moiety of ubiquinone (Coenzyme Q) in Arabidopsis. Plant Cell 26:1938–1948
Bulgakov VP, Inyushkina YV, Fedoreyev SA (2012) Rosmarinic acid and its derivatives: biotechnology and applications. Crit Rev Biotechnol 32:203–217
Cass CL, Peraldi A, Dowd PF, Mottiar Y, Santoro N, Karlen SD, Bukhman YV, Foster CE, Thrower N, Bruno LC, Moskvin OV, Johnson ET, Willhoit ME, Phutane M, Ralph J, Mansfield SD, Nicholson P, Sedbrook JC (2015) Effects of PHENYLALANINE AMMONIA LYASE (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachypodium. J Exp Bot 66:4317–4335
Cassels BK, Saez-Briones P (2018) Dark classics in chemical neuroscience: mescaline. ACS Chem Neurosci 9:2448–2458
Christou P, Barton KA (1989) Cytokinin antagonist activity of substituted phenethylamines in plant cell culture. Plant Physiol 89:564–568
Clausen M, Kannangara RM, Olsen CE, Blomstedt CK, Gleadow RM, Jorgensen K, Bak S, Motawie MS, Moller BL (2015) The bifurcation of the cyanogenic glucoside and glucosinolate biosynthetic pathways. Plant J 84:558–573
Ehrenworth AM, Peralta-Yahya P (2017) Accelerating the semisynthesis of alkaloid-based drugs through metabolic engineering. Nat Chem Biol 13:249–258
Facchini PJ, De Luca V (1994) Differential and tissue-specific expression of a gene family for tyrosine/dopa decarboxylase in opium poppy. J Biol Chem 269:26684–26690
Facchini PJ, De Luca V (1995) Expression in Escherichia coli and partial characterization of two tyrosine/dopa decarboxylases from opium poppy. Phytochemistry 38:1119–1126
Facchini PJ, Huber-Allanach KL, Tari LW (2000) Plant aromatic l-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry 54:121–138
Facchini PJ, Hagel J, Zulak KG (2002) Hydroxycinnamic acid amide metabolism: physiology and biochemistry. Can J Bot 80:577–589
Falk J, Munne-Bosch S (2010) Tocochromanol functions in plants: antioxidation and beyond. J Exp Bot 61:1549–1566
Fritsche S, Wang X, Jung C (2017) Recent advances in our understanding of tocopherol biosynthesis in plants: an overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants (Basel) 6:99
Goodstein DM, Shu S, Howson R, Neupane R, Hayes RD, Fazo J, Mitros T, Dirks W, Hellsten U, Putnam N, Rokhsar DS (2012) Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res 40:D1178–1186
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321
Hatlestad GJ, Sunnadeniya RM, Akhavan NA, Gonzalez A, Goldman IL, McGrath JM, Lloyd AM (2012) The beet R locus encodes a new cytochrome P450 required for red betalain production. Nat Genet 44:816–820
Häusler E, Petersen M, Alfermann AW (1991) Hydroxyphenylpyruvate reductase from cell suspension cultures of Coleus blumei Benth. Zeitschrift für Naturforschung C 46:371–376
Horvath G, Wessjohann L, Bigirimana J, Jansen M, Guisez Y, Caubergs R, Horemans N (2006) Differential distribution of tocopherols and tocotrienols in photosynthetic and non-photosynthetic tissues. Phytochemistry 67:1185–1195
Jones PR, Moller BL, Hoj PB (1999) The UDP-glucose: p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor. Isolation, cloning, heterologous expression, and substrate specificity. J Biol Chem 274:35483–35491
Jun SY, Sattler SA, Cortez GS, Vermerris W, Sattler SE, Kang C (2018) Biochemical and structural analysis of substrate specificity of a Phenylalanine Ammonia-Lyase. Plant Physiol 176:1452–1468
Kahn RA, Bak S, Svendsen I, Halkier BA, Moller BL (1997) Isolation and reconstitution of cytochrome P450ox and in vitro reconstitution of the entire biosynthetic pathway of the cyanogenic glucoside dhurrin from sorghum. Plant Physiol 115:1661–1670
Kang S, Kang K, Lee K, Back K (2007) Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta 227:263–272
Kilgore MB, Kutchan TM (2016) The Amaryllidaceae alkaloids: biosynthesis and methods for enzyme discovery. Phytochem Rev 15:317–337
Koch BM, Sibbesen O, Halkier BA, Svendsen I, Moller BL (1995) The primary sequence of cytochrome P450tyr, the multifunctional N-hydroxylase catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys 323:177–186
Kulma A, Szopa J (2007) Catecholamines are active compounds in plants. Plant Sci 172:433–440
Kyndt JA, Meyer TE, Cusanovich MA, Van Beeumen JJ (2002) Characterization of a bacterial tyrosine ammonia lyase, a biosynthetic enzyme for the photoactive yellow protein. FEBS Lett 512:240–244
Laursen T, Borch J, Knudsen C, Bavishi K, Torta F, Martens HJ, Silvestro D, Hatzakis NS, Wenk MR, Dafforn TR, Olsen CE, Motawia MS, Hamberger B, Moller BL, Bassard JE (2016) Characterization of a dynamic metabolon producing the defense compound dhurrin in sorghum. Science 354:890–893
Lee EJ, Facchini PJ (2011) Tyrosine aminotransferase contributes to benzylisoquinoline alkaloid biosynthesis in opium poppy. Plant Physiol 157:1067–1078
Lehmann T, Pollmann S (2009) Gene expression and characterization of a stress-induced tyrosine decarboxylase from Arabidopsis thaliana. FEBS Lett 583:1895–1900
Liscombe DK, Macleod BP, Loukanina N, Nandi OI, Facchini PJ (2005) Evidence for the monophyletic evolution of benzylisoquinoline alkaloid biosynthesis in angiosperms. Phytochemistry 66:1374–1393
Liu M, Lu S (2016) Plastoquinone and ubiquinone in plants: biosynthesis, physiological function and metabolic engineering. Front Plant Sci 7:1–18
Liu X, Liu Y, Huang P, Ma Y, Qing Z, Tang Q, Cao H, Cheng P, Zheng Y, Yuan Z, Zhou Y, Liu J, Tang Z, Zhuo Y, Zhang Y, Yu L, Huang J, Yang P, Peng Q, Zhang J, Jiang W, Zhang Z, Lin K, Ro DK, Chen X, Xiong X, Shang Y, Huang S, Zeng J (2017) The genome of medicinal plant Macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol Plant 10:975–989
Louie GV, Bowman ME, Moffitt MC, Baiga TJ, Moore BS, Noel JP (2006) Structural determinants and modulation of substrate specificity in phenylalanine-tyrosine ammonia-lyases. Chem Biol 13:1327–1338
Maeda H, Dudareva N (2012) The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol 63:73–105
Meiser J, Weindl D, Hiller K (2013) Complexity of dopamine metabolism. Cell Commun Signal 11:34
Mene-Saffrane L (2017) Vitamin E biosynthesis and its regulation in plants. Antioxidants (Basel) 7:2
Moran GR (2005) 4-Hydroxyphenylpyruvate dioxygenase. Arch Biochem Biophys 433:117–128
Munné-Bosch S, Alegre L (2002) The function of tocopherols and tocotrienols in Plants. Crit Rev Plant Sci 21:31–57
Ndikuryayo F, Moosavi B, Yang WC, Yang GF (2017) 4-Hydroxyphenylpyruvate dioxygenase inhibitors: from chemical biology to agrochemicals. J Agric Food Chem 65:8523–8537
Negrel J, Javelle F, Paynot M (1993) Biochemical basis of resistance of tobacco callus tissue cultures to hydroxyphenylethylamines. Plant Physiol 103:329–334
Nielsen KA, Tattersall DB, Jones PR, Moller BL (2008) Metabolon formation in dhurrin biosynthesis. Phytochemistry 69:88–98
Nomura T, Kutchan TM (2010) Three new O-methyltransferases are sufficient for all O-methylation reactions of ipecac alkaloid biosynthesis in root culture of Psychotria ipecacuanha. J Biol Chem 285:7722–7738
Norris SR, Barrette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7:2139–2149
Norris SR, Shen X, DellaPenna D (1998) Complementation of the Arabidopsis pds1 mutation with the gene encoding p-hydroxyphenylpyruvate dioxygenase. Plant Physiol 117:1317–1323
Payet LA, Leroux M, Willison JC, Kihara A, Pelosi L, Pierrel F (2016) Mechanistic details of early steps in Coenzyme Q biosynthesis pathway in yeast. Cell Chem Biol 23:1241–1250
Petersen M (2013) Rosmarinic acid: new aspects. Phytochem Rev 12:207–227
Petersen M, Alfermann AW (1988) Two new enzymes of rosmarinic acid biosynthesis from cell cultures of Coleus blumei: hydroxyphenylpyruvate reductase and rosmarinic acid synthase. Zeitschrift für Naturforschung C 43:501–504
Petersen M, Simmonds MS (2003) Rosmarinic acid. Phytochemistry 62:121–125
Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hucherig S, Janiak V, Kim KH, Sander M, Weitzel C, Wolters S (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70:1663–1679
Polturak G, Aharoni A (2018) “La Vie en Rose”: biosynthesis, sources, and applications of betalain pigments. Mol Plant 11:7–22
Polturak G, Breitel D, Grossman N, Sarrion-Perdigones A, Weithorn E, Pliner M, Orzaez D, Granell A, Rogachev I, Aharoni A (2016) Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytol 210:269–283
Pravst I, Zmitek K, Zmitek J (2010) Coenzyme Q10 contents in foods and fortification strategies. Crit Rev Food Sci Nutr 50:269–280
Riewe D, Koohi M, Lisec J, Pfeiffer M, Lippmann R, Schmeichel J, Willmitzer L, Altmann T (2012) A tyrosine aminotransferase involved in tocopherol synthesis in Arabidopsis. Plant J 71:850–859
Rinner U, Waser M (2016) Tyrosine alkaloids. In: Zografos AL (ed) From biosynthesis to total synthesis: strategies and tactics for natural products, pp 431–472
Rippert P, Scimemi C, Dubald M, Matringe M (2004) Engineering plant shikimate pathway for production of tocotrienol and improving herbicide resistance. Plant Physiol 134:92–100
Rosler J, Krekel F, Amrhein N, Schmid J (1997) Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol 113:175–179
Ru M, Wang K, Bai Z, Peng L, He S, Wang Y, Liang Z (2017) A tyrosine aminotransferase involved in rosmarinic acid biosynthesis in Prunella vulgaris L. Scientific Reports. 7:4892
Schenck CA, Maeda HA (2018) Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 149:82–102
Schenck CA, Chen S, Siehl DL, Maeda HA (2015) Non-plastidic, tyrosine-insensitive prephenate dehydrogenases from legumes. Nat Chem Biol 11:52–57
Schlager S, Drager B (2016) Exploiting plant alkaloids. Curr Opin Biotechnol 37:155–164
Shukla S, Dubey KK (2018) CoQ10 a super-vitamin: review on application and biosynthesis. 3 Biotech 8:249
Sibbesen O, Koch B, Halkier BA, Moller BL (1995) Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of l-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem 270:3506–3511
Soubeyrand E, Johnson TS, Latimer S, Block A, Kim J, Colquhoun TA, Butelli E, Martin C, Wilson MA, Basset GJ (2018) The peroxidative cleavage of kaempferol contributes to the biosynthesis of the benzenoid moiety of ubiquinone in plants. Plant Cell 30:2910–2921
Stacey MG, Cahoon RE, Nguyen HT, Cui Y, Sato S, Nguyen CT, Phoka N, Clark KM, Liang Y, Forrester J, Batek J, Do PT, Sleper DA, Clemente TE, Cahoon EB, Stacey G (2016) Identification of homogentisate dioxygenase as a target for vitamin E biofortification in oilseeds. Plant Physiol 172:1506–1518
Stefely JA, Kwiecien NW, Freiberger EC, Richards AL, Jochem A, Rush MJP, Ulbrich A, Robinson KP, Hutchins PD, Veling MT, Guo X, Kemmerer ZA, Connors KJ, Trujillo EA, Sokol J, Marx H, Westphall MS, Hebert AS, Pagliarini DJ, Coon JJ (2016) Mitochondrial protein functions elucidated by multi-omic mass spectrometry profiling. Nat Biotechnol 34:1191–1197
Sunnadeniya R, Bean A, Brown M, Akhavan N, Hatlestad G, Gonzalez A, Symonds VV, Lloyd A (2016) Tyrosine hydroxylation in betalain pigment biosynthesis is performed by cytochrome P450 enzymes in beets (Beta vulgaris). PLoS One 11:1–16
Tattersall DB, Bak S, Jones PR, Olsen CE, Nielsen JK, Hansen ML, Hoj PB, Moller BL (2001) Resistance to an herbivore through engineered cyanogenic glucoside synthesis. Science 293:1826–1828
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Torrens-Spence MP, Gillaspy G, Zhao B, Harich K, White RH, Li J (2012) Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme. Biochem Biophys Res Commun 418:211–216
Torrens-Spence MP, Liu P, Ding H, Harich K, Gillaspy G, Li J (2013) Biochemical evaluation of the decarboxylation and decarboxylation-deamination activities of plant aromatic amino acid decarboxylases. J Biol Chem 288:2376–2387
Torrens-Spence MP, Lazear M, von Guggenberg R, Ding H, Li J (2014) Investigation of a substrate-specifying residue within Papaver somniferum and Catharanthus roseus aromatic amino acid decarboxylases. Phytochemistry 106:37–43
Torrens-Spence MP, Pluskal T, Li FS, Carballo V, Weng JK (2018a) Complete pathway elucidation and heterologous reconstitution of rhodiola salidroside biosynthesis. Mol Plant 11:205–217
Torrens-Spence MP, Chiang Y-C, Smith T, Vicent MA, Wang Y, Weng J-K (2018b) Structural basis for independent origins of new catalytic machineries in plant AAAD proteins. bioRxiv 404970
Tran LT, Taylor JS, Constabel CP (2012) The polyphenol oxidase gene family in land plants: lineage-specific duplication and expansion. BMC Genom 13:395
Van Bel M, Diels T, Vancaester E, Kreft L, Botzki A, Van de Peer Y, Coppens F, Vandepoele K (2018) PLAZA 4.0: an integrative resource for functional, evolutionary and comparative plant genomics. Nucleic Acids Res 46:D1190–D1196
Wang MM, Maeda HA (2018) Aromatic amino acid aminotransferases in plants. Phytochem Rev 17:131–159
Wang M, Toda K, Maeda HA (2016) Biochemical properties and subcellular localization of tyrosine aminotransferases in Arabidopsis thaliana. Phytochemistry 132:16–25
Wang M, Toda K, Block A, Maeda HA (2019) TAT1 and TAT2 tyrosine aminotransferases have both distinct and shared functions in tyrosine metabolism and degradation in Arabidopsis thaliana. J Biol Chem 294:3563–3576
Watts KT, Mijts BN, Lee PC, Manning AJ, Schmidt-Dannert C (2006) Discovery of a substrate selectivity switch in tyrosine ammonia-lyase, a member of the aromatic amino acid lyase family. Chem Biol 13:1317–1326
Wiegrebe W, Kramer WJ, Shamma M (1984) The emetine alkaloids. J Nat Prod 47:397–408
Wu YB, Ni ZY, Shi QW, Dong M, Kiyota H, Gu YC, Cong B (2012) Constituents from Salvia species and their biological activities. Chem Rev 112:5967–6026
Xu JJ, Fang X, Li CY, Zhao Q, Martin C, Chen XY, Yang L (2018) Characterization of Arabidopsis thaliana hydroxyphenylpyruvate reductases in the tyrosine conversion pathway. Front Plant Sci 9:1305
Zhang C, Cahoon RE, Hunter SC, Chen M, Han J, Cahoon EB (2013) Genetic and biochemical basis for alternative routes of tocotrienol biosynthesis for enhanced vitamin E antioxidant production. Plant J 73:628–639
Acknowledgements
This project was supported by the Special Fund for Shanghai Landscaping Administration Bureau Program (Grant nos. G192416 and G192419).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, JJ., Fang, X., Li, CY. et al. General and specialized tyrosine metabolism pathways in plants. aBIOTECH 1, 97–105 (2020). https://doi.org/10.1007/s42994-019-00006-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42994-019-00006-w