Abstract
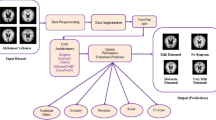
Alzheimer disease (AD) is a chronic neurological disorder in which the loss of brain cells causes dementia. Early and accurate diagnosis of AD will lead to better treatment of the disease before irreversible brain damage has been occurred. This paper proposes the classification of Alzheimer's disease using 3D structural Magnetic Resonance Imaging (sMRI) images through 3D convolutional neural networks (CNNs). Most existing methods utilizing 3D subject-level CNNs for Alzheimer's disease classification design a single model which relies on a very large training dataset for improved generalization. Herein, we address this issue through 3D transfer learning which makes use of knowledge gained from a pre-trained task. We train 3D versions of five classical 2D image classification architectures—ResNet, ResNeXt, SE-ResNet, SE-ResNeXt, and SE-Net—by initializing each model with pre-trained weights from their 2D counterparts, and combine their predictions through a weighted average method. The weights assigned to each model of the ensemble are optimized to achieve a performance better than any single 3D CNN model. With a relatively smaller training dataset, the proposed model obtains 97.27%, 82.33%, 90.41%, 84.22%, 84.26%, and 77.1% accuracies for the Alzheimer’s disease (AD) versus cognitively normal (CN), early mild cognitive impairment (EMCI) versus CN, late mild cognitive impairment (LMCI) versus CN, EMCI versus AD, LMCI versus AD, and EMCI versus LMCI classification tasks, outperforming current state-of-the-art methods, and indicating the effectiveness of our proposed model.











Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Ebrahimighahnavieh MA, Luo S, Chiong R. Deep learning to detect Alzheimer’s disease from neuroimaging: a systematic literature review. Comput Methods Programs Biomed. 2020;187:105242.
Aisen PS, Petersen RC, Donohue MC, Gamst A, Raman R, Thomas RG, Walter S, Trojanowski JQ, Shaw LM, Beckett LA, Jack CR Jr. Clinical core of the Alzheimer’s disease neuroimaging initiative: progress and plans. Alzheimers Dement. 2010;6(3):239–46.
Prince M, Bryce R, Ferri C. World Alzheimer report 2011: the benefits of early diagnosis and intervention; 2011. https://www.alzint.org/u/WorldAlzheimerReport2011.pdf.
Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P, Lautenschlager NT, Lenzo N, Martins RN, Maruff P, Masters C. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int Psychogeriatr. 2009;21(4):672–87.
Marcus DS, Wang TH, Parker J, Csernansky JG, Morris JC, Buckner RL. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J Cogn Neurosci. 2007;19(9):1498–507.
Bron EE, Smits M, Van Der Flier WM, Vrenken H, Barkhof F, Scheltens P, Papma JM, Steketee RM, Orellana CM, Meijboom R, Pinto M. Standardized evaluation of algorithms for computer-aided diagnosis of dementia based on structural MRI: the CADDementia challenge. Neuroimage. 2015;111:562–79.
Deng J, Dong W, Socher R, Li LJ, Li K, Fei-Fei L. Imagenet: a large-scale hierarchical image database. In: IEEE conference on computer vision and pattern recognition; 2009. p. 248–55.
Kay W, Carreira J, Simonyan K, Zhang B, Hillier C, Vijayanarasimhan S, Viola F, Green T, Back T, Natsev P, Suleyman M. The kinetics human action video dataset. Preprint arXiv:1705.06950; 2017.
Kuehne H, Jhuang H, Garrote E, Poggio T, Serre T. HMDB: a large video database for human motion recognition. In: International conference on computer vision; 2011. p. 2556–63.
Soomro K, Zamir AR, Shah M. UCF101: a dataset of 101 human actions classes from videos in the wild. Preprint arXiv:1212.0402; 2012.
Hara K, Kataoka H, Satoh Y. Learning spatio-temporal features with 3d residual networks for action recognition. In: IEEE international conference on computer vision workshops; 2017. p. 3154–60.
Carreira J, Zisserman A. Quo vadis, action recognition? A new model and the kinetics dataset. In: IEEE Conference on Computer Vision and Pattern Recognition; 2017. p. 6299–308.
He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. In: IEEE conference on computer vision and pattern recognition; 2016. p. 770–8.
Xie S, Girshick R, Dollár P, Tu Z, He K. Aggregated residual transformations for deep neural networks. In: IEEE conference on computer vision and pattern recognition; 2017. p. 1492–500.
Hu J, Shen L, Sun G. Squeeze-and-excitation networks. In: IEEE conference on computer vision and pattern recognition; 2018. p. 7132–41.
Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. Preprint arXiv:1409.1556; 2014.
Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, Erhan D, Vanhoucke V, Rabinovich A. Going deeper with convolutions. In: IEEE conference on computer vision and pattern recognition; 2015. p. 1–9.
Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. Adv Neural Inform Process syst. 2012;25:1.
Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. In: IEEE conference on computer vision and pattern recognition; 2017. p. 4700–08.
Szegedy C, Ioffe S, Vanhoucke V, Alemi AA. Inception-v4, inception-resnet and the impact of residual connections on learning. In: Thirty-first AAAI conference on artificial intelligence; 2017.
Brownlee J. Ensemble learning methods for deep learning neural networks. Machine Learning Mastery; 2018. https://machinelearningmastery.com/ensemble-methods-for-deep-learning-neural-networks/. Accessed: 03-Jan-2022.
Yan WQ. Computational methods for deep learning. London: Springer; 2021.
Seni G, Elder JF. Ensemble methods in data mining: improving accuracy through combining predictions. Synth Lect Data Min Knowl Disc. 2010;2(1):1–26.
Boggs PT, Tolle JW. Sequential quadratic programming. Acta Numer. 1995;4:1–51.
Wen J, Thibeau-Sutre E, Diaz-Melo M, Samper-González J, Routier A, Bottani S, Dormont D, Durrleman S, Burgos N, Colliot O. Alzheimer’s Disease Neuroimaging Initiative. Convolutional neural networks for classification of Alzheimer’s disease: overview and reproducible evaluation. Med image Anal. 2020;63:101694.
Korolev S, Safiullin A, Belyaev M, Dodonova Y. Residual and plain convolutional neural networks for 3D brain MRI classification. In: 2017 IEEE 14th international symposium on biomedical imaging (ISBI 2017). IEEE; Apr-2017.
Korolev S, Safiullin A, Belyaev M, Dodonova Y. Residual and plain convolutional neural networks for 3D brain MRI classification. In: IEEE 14th international symposium on biomedical imaging (ISBI 2017); 2017. p. 835–8.
Bae JB, Lee S, Jung W, Park S, Kim W, Oh H, Han JW, Kim GE, Kim JS, Kim JH, Kim KW. Identification of Alzheimer’s disease using a convolutional neural network model based on T1-weighted magnetic resonance imaging. Sci Rep. 2020;10(1):1.
Ebrahimi A, Luo S, Chiong R. Introducing transfer learning to 3D ResNet-18 for Alzheimer’s disease detection on MRI images. In: 35th international conference on image and vision computing New Zealand (IVCNZ); 2020. p. 1–6.
Oh K, Chung YC, Kim KW, Kim WS, Oh IS. Classification and visualization of Alzheimer’s disease using volumetric convolutional neural network and transfer learning. Sci Rep. 2019;9(1):1–6.
Folego G, Weiler M, Casseb RF, Pires R, Rocha A. Alzheimer’s disease detection through whole-brain 3D-CNN MRI. Front Bioeng Biotechnol. 2020;8:534592.
Pan D, Zeng A, Jia L, Huang Y, Frizzell T, Song X. Early detection of Alzheimer’s disease using magnetic resonance imaging: a novel approach combining convolutional neural networks and ensemble learning. Front Neurosci. 2020;14:259.
Islam J, Zhang Y. Brain MRI analysis for Alzheimer’s disease diagnosis using an ensemble system of deep convolutional neural networks. Brain Inform. 2018;5(2):1–4.
Choi JY, Lee B. Combining of multiple deep networks via ensemble generalization loss, based on MRI images, for Alzheimer’s disease classification. IEEE Signal Process Lett. 2020;27:206–10.
Xing X, Liang G, Blanton H, Rafique MU, Wang C, Lin AL, Jacobs N. Dynamic image for 3D MRI image Alzheimer’s disease classification. In: European conference on computer vision; 2020. p. 355–64.
Xia Z, Yue G, Xu Y, Feng C, Yang M, Wang T, Lei B. A novel end-to-end hybrid network for Alzheimer's disease detection using 3D CNN and 3D CLSTM. In: IEEE 17th international symposium on biomedical imaging (ISBI); 2020. p. 1–4.
Liu M, Zhang J, Adeli E, Shen D. Landmark-based deep multi-instance learning for brain disease diagnosis. Med Image Anal. 2018;43:157–68.
Hong X, Lin R, Yang C, Zeng N, Cai C, Gou J, Yang J. Predicting Alzheimer’s disease using LSTM. IEEE Access. 2019;7:80893–901.
Islam J, Zhang Y. A novel deep learning based multi-class classification method for Alzheimer’s disease detection using brain MRI data. In: International conference on brain informatics; 2017. p. 213–22.
Hon M, Khan NM. Towards Alzheimer's disease classification through transfer learning. In: IEEE international conference on bioinformatics and biomedicine (BIBM); 2017. p. 1166–9.
Wang S, Shen Y, Chen W, Xiao T, Hu J. Automatic recognition of mild cognitive impairment from MRI images using expedited convolutional neural networks. In: International conference on artificial neural networks; 2017. p. 373–80.
Farooq A, Anwar S, Awais M, Rehman S. A deep CNN based multi-class classification of Alzheimer's disease using MRI. In: IEEE international conference on imaging systems and techniques (IST); 2017. p. 1–6.
Pérez-García F, Sparks R, Ourselin S. TorchIO: a Python library for efficient loading, preprocessing, augmentation and patch-based sampling of medical images in deep learning. Comput Methods Programs Biomed. 2021;208:106236.
Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, Burovski E, Peterson P, Weckesser W, Bright J, Van Der Walt SJ. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17(3):261–72.
Dharwada S, Tembhurne J, Diwan T. Multi-channel deep model for classification of Alzheimer’s disease using transfer learning. In: International conference on distributed computing and internet technology; 2022. p. 245–59.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dharwada, S., Tembhurne, J. & Diwan, T. An Optimal Weighted Ensemble of 3D CNNs for Early Diagnosis of Alzheimer’s Disease. SN COMPUT. SCI. 5, 252 (2024). https://doi.org/10.1007/s42979-023-02581-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42979-023-02581-8