Abstract
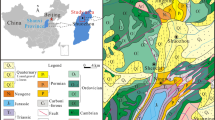
With over 260 tons of gold reserves, the Shuiyindong gold mine is one of the largest Carlin-type gold deposits in China. A particular challenge in the processing of the ore is the presence of carbonaceous compounds, which can cause substantial losses in recoveries via the preg-robbing and adsorption of gold. To investigate the structural properties of the native carbon and to compare between different characterization techniques for such compounds, pre- and post-flotation mineral samples containing 0.9–4.8% non-carbonate carbon from the Shuiyindong mine have been examined via mineralogical and thermogravimetric approaches, as well as spectroscopic techniques, including X-ray photoelectron spectroscopy (XPS), near-edge X-ray absorption fine edge structure spectroscopy (NEXAFS), solid-state 13C nuclear magnetic resonance (NMR) spectroscopy, diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy, Raman spectroscopy and preg-robbing measurements. NEXAFS and DRIFT yielded the most reliable results, indicating that 60–75% of the organic carbon in all samples consist of aliphatic alkenes with substantial unsaturated content, highly similar to activated carbon, with the remainder being graphitic and humic. Speciation by XPS was sub-optimal due to spectral overlaps between graphitic and aliphatic carbon, and between carboxylic and carbonate functionalities, while NMR spectroscopy could not provide reliable speciation of the carbon groups. The preg-robbing strength was strongly correlated to the aliphatic content and structural disorder in the carbon, while no such relationship was found with the graphitic and humic content.








Similar content being viewed by others
References
Tan Q-P, Xia Y, Xie Z-J, Yan J (2015) Migration paths and precipitation mechanisms of ore-forming fluids at the Shuiyindong Carlin-type gold deposit, Guizhou, China. Ore Geol Rev 69:140–156. https://doi.org/10.1016/j.oregeorev.2015.02.006
Tan Q-P, Xia Y, Xie Z-J, Yan J, Wei D (2015) S, C, O, H, and Pb isotopic studies for the Shuiyindong Carlin-type gold deposit, Southwest Guizhou, China: constraints for ore genesis. Chin J Geochem 34:525–539. https://doi.org/10.1007/s11631-015-0063-5
Li X, Zhang Q, Shen Z (2016) Study of the separation of carbonaceous matter in micro-grained gold ore in Guizhou Province. Nonferrous Metals 3:33–37,55
Su W, Zhang H, Hu R, Ge X, Xia B, Chen Y, Zhu C (2012) Mineralogy and geochemistry of gold-bearing arsenian pyrite from the Shuiyindong Carlin-type gold deposit, Guizhou, China: implications for gold depositional processes. Mineral Deposita 47:653–662. https://doi.org/10.1007/s00126-011-0328-9
Ashley R, Cunningham C, Bostick N, Dean W, Chou I-M (1991) Geology and geochemistry of three sedimentary-rock-hosted disseminated gold deposits in Guizhou Province, People's Republic of China. Ore Geol Rev 6:133–151
Wang J, Chen J, Xiong M, Cai C, Li J, Yang H (2018) Treatment of refractory gold ores in China. In: ALTA 2018 Gold-PM conference. ALTA Metallurgical Service, Perth
Marsden J, House I (2006) The chemistry of gold extraction. Society for Mining, metallurgy, and Exploration, Littleton, Colorado
Miller J, Wan RY, Díaz X (2016) Chapter 49 - Preg-robbing gold ores. In: Gold ore processing (second edn). pp 885–907. https://doi.org/10.1016/B978-0-444-63658-4.00049-9
Ng WS, Wang Q, Chen M (2020) A review of preg-robbing and the impact of chloride ions in the pressure oxidation of double refractory ores. Miner Process Extr Metall Rev. https://doi.org/10.1080/08827508.2020.1793142
Helm M, Vaughan J, Staunton WP, Avraamides J (2009) An investigation of the carbonaceous component of preg-robbing gold ores. Paper presented at the World Gold Conference 2009, Cape Town, 14–18 April
Stenebråten JF, Johnson W, McMullen J (2000) Characterization of Goldstrike ore carbonaceous material. Part 2: physical characteristics. Min Metall Explor 17:7–15. https://doi.org/10.1007/BF03402823
Stenebråten JF, Johnson W, Brosnahan DR (1999) Characterization of Goldstrike ore carbonaceous material. Part 1: chemical characteristics. Min Metall Explor 16:37–43. https://doi.org/10.1007/BF03402817
Yang H-y, Liu Q, Song X-l, Dong J-k (2013) Research status of carbonaceous matter in carbonaceous gold ores and bio-oxidation pretreatment. Trans Nonferrous Metals Soc China 23:3405–3411. https://doi.org/10.1016/S1003-6326(13)62881-2
Radtke AS, Scheiner BJ (1970) Studies of hydrothermal gold deposition (I). Carlin gold deposit, Nevada the role of carbonaceous materials in gold deposition. Econ Geol 65:87–102. https://doi.org/10.2113/gsecongeo.65.2.87
Augustyniak-Jabłokow MA, Yablokov YV, Andrzejewski B, Kempiński W, Łoś S, Tadyszak K, Yablokov MY, Zhikharev VA (2010) EPR and magnetism of the nanostructured natural carbonaceous material shungite. Phys Chem Miner 37:237–247. https://doi.org/10.1007/s00269-009-0328-9
Tan H, Feng D, Lukey GC, van Deventer JSJ (2005) The behaviour of carbonaceous matter in cyanide leaching of gold. Hydrometallurgy 78:226–235. https://doi.org/10.1016/j.hydromet.2005.03.001
Pourdasht M, Xia L, Dimov S, Hart B, Chen Z (2017) Preg-robbing carbonaceous matter: an evaluation of surface chemical control. Paper presented at the COM2017, Vancouver, Canada, 27–30 August
Singh B (2014) NEXAFS and XPS characterisation of carbon functional groups of fresh and aged biochars. Org Geochem 77:1–10. https://doi.org/10.1016/j.orggeochem.2014.09.006
Braun A, Huggins FE, Shah N, Chen Y, Wirick S, Mun SB, Jacobsen C, Huffman GP (2005) Advantages of soft X-ray absorption over TEM-EELS for solid carbon studies––a comparative study on diesel soot with EELS and NEXAFS. Carbon 43:117–124. https://doi.org/10.1016/j.carbon.2004.08.029
Ofori-Sarpong G, Amankwah RK, Osseo-Asare K (2013) Reduction of preg-robbing by biomodified carbonaceous matter – a proposed mechanism. Miner Eng 42:29–35. https://doi.org/10.1016/j.mineng.2012.11.014
Suggate RP, Dickinson WW (2004) Carbon NMR of coals: the effects of coal type and rank. Int J Coal Geol 57:1–22. https://doi.org/10.1016/S0166-5162(03)00116-2
Clough A, Sigle JL, Jacobi D, Sheremata J, White JL (2015) Characterization of kerogen and source rock maturation using solid-state NMR spectroscopy. Energy Fuel 29:6370–6382. https://doi.org/10.1021/acs.energyfuels.5b01669
Preston CM (1996) Applications of NMR to soil organic matter analysis: history and prospects. Soil Sci 161:144–166
Chukov SN, Lodygin ED, Abakumov EV (2018) Application of 13C NMR spectroscopy to the study of soil organic matter: a review of publications. Eurasian Soil Sci 51:889–900. https://doi.org/10.1134/S1064229318080021
Konadu KT, Sasaki K, Kaneta T, Ofori-Sarpong G, Osseo-Asare K (2017) Bio-modification of carbonaceous matter in gold ores: model experiments using powdered activated carbon and cell-free spent medium of Phanerochaete chrysosporium. Hydrometallurgy 168:76–83. https://doi.org/10.1016/j.hydromet.2016.08.003
Begaudeau K, Morizet Y, Florian P, Paris M, Mercier J-C (2012) Solid-state NMR analysis of Fe-bearing minerals: implications and applications for earth sciences. Eur J Mineral 24:535–550
Margenot AJ, Calderón FJ, Goyne KW, Dmukome FN, Parikh S (2016) IR spectroscopy, soil analysis applications. In: Encyclopedia of Spectroscopy and Spectrometry. Elsevier, pp. 448–454
Haberhauer G, Gerzabek M (1999) Drift and transmission FT-IR spectroscopy of forest soils: an approach to determine decomposition processes of forest litter. Vib Spectrosc 19:413–417
Calderón FJ, Mikha MM, Vigil MF, Nielsen DC, Benjamin JG, Reeves JB III (2011) Diffuse-reflectance mid-infrared spectral properties of soils under alternative crop rotations in a semi-arid climate. Commun Soil Sci Plant Anal 42:2143–2159
Dunne R, Buda K, Hill M, Staunton W, Wardell-Johnson G, Tjandrawan V (2012) Assessment of options for economic processing of preg-robbing gold ores. Miner Process Extr M 121:217–223. https://doi.org/10.1179/1743285512Y.0000000019
Dunne R, Staunton WP, Afewu K (2013) A historial review of the treatment of preg-robbing gold ores - what has worked and changed. In: World gold 2013, Brisbane, Australia. AusIMM, Burwood, Victoria, Australia, pp. 99–110
Helm M, Vaughan J, Staunton W (2012) Evaluation of preg-robbing in Goldstrike carbonaceous ore using Raman spectroscopy. In: 50th conference of metallurgists. Montreal, Quebec, 2–5 Oct 2011, pp 595–606
Ren Z, Zhang T, Liu J, Tomlinson M, Asselin E (2017) Characterization of carbonaceous matters associated with preg-robbing ores. Paper presented at the COM2017, Vancouver, Canada, 27–30 August
Shirley DA (1972) High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys Rev B 5:4709–4714. https://doi.org/10.1103/PhysRevB.5.4709
Ravel B, Newville M (2005) ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J Synchrotron Radiat 12:537–541
Batson PE (1993) Carbon 1s near-edge-absorption fine structure in graphite. Phys Rev B 48:2608–2610. https://doi.org/10.1103/PhysRevB.48.2608
Lorenzen L, Van Deventer JSJ (1992) The mechanism of leaching of gold from refractory ores. Miner Eng 5:1377–1387. https://doi.org/10.1016/0892-6875(92)90173-7
Celep O, Alp İ, Devecİ H, Vicil M (2009) Characterization of refractory behaviour of complex gold/silver ore by diagnostic leaching. Trans Nonferrous Metals Soc China 19:707–713. https://doi.org/10.1016/S1003-6326(08)60337-4
Liu Q, Yang H-y, Tong L-l (2014) Influence of Phanerochaete chrysosporium on degradation and preg-robbing capacity of activated carbon. Trans Nonferrous Metals Soc China 24:1905–1911. https://doi.org/10.1016/S1003-6326(14)63270-2
Földvári M (2011) Handbook of thermogravimetric system of minerals and its use in geological practice. Geological Institute of Hungary (=Magyar Állami Földtani Intézet), Budapest, Hungary
Hammerschmidt J, Güntner J, Kerstiens B, Charitos A (2016) Chapter 24 - roasting of gold ore in the circulating fluidized-bed technology. In: Adams MD (ed) Gold ore processing, 2nd edn. Elsevier, Amsterdam, pp 393–409. https://doi.org/10.1016/B978-0-444-63658-4.00024-4
Nielsen L, Biggs MJ, Skinner W, Bandosz TJ (2014) The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 80:419–432. https://doi.org/10.1016/j.carbon.2014.08.081
Dedryvère R, Gireaud L, Grugeon S, Laruelle S, Tarascon JM, Gonbeau D (2005) Characterization of lithium alkyl carbonates by X-ray photoelectron spectroscopy: experimental and theoretical study. J Phys Chem B 109:15868–15875. https://doi.org/10.1021/jp051626k
Kara F, AdigÜzel D, Atmaca U, Çelİk M, Naktİyok J (2020) Characterization and kinetics analysis of the thermal decomposition of the humic substance from hazelnut husk. Turk J Chem 44:1483–1494. https://doi.org/10.3906/kim-2004-62
Rotaru A, Nicolaescu I, Rotaru P, Neaga C (2008) Thermal characterization of humic acids and other components of raw coal. J Therm Anal Calorim 92:297–300
Janoš P, Kozler J (1995) Thermal stability of humic acids and some of their derivatives. Fuel 74:708–713. https://doi.org/10.1016/0016-2361(94)00007-E
Brandes JA, Wirick S, Jacobsen C (2010) Carbon K-edge spectra of carbonate minerals. J Synchrotron Radiat 17:676–682
Liu LZ, Nie ZY, Yang Y, Pan X, Xia X, Zhou YH, Xia JL, Zhang LJ, Zhen XJ, Yang HY (2018) In situ characterization of change in superficial organic components of thermoacidophilic archaeon Acidianus manzaensis YN-25. Res Microbiol 169:590–597. https://doi.org/10.1016/j.resmic.2018.08.003
Freitas JC, Cipriano DF, Zucolotto CG, Cunha AG, Emmerich FG (2016) Solid-state 13C NMR spectroscopy applied to the study of carbon blacks and carbon deposits obtained by plasma pyrolysis of natural gas. J Spectrosc 2016. https://doi.org/10.1155/2016/1543273
Freitas JCC, Emmerich FG, Cernicchiaro GRC, Sampaio LC, Bonagamba TJ (2001) Magnetic susceptibility effects on 13C MAS NMR spectra of carbon materials and graphite. Solid State Nucl Magn Reson 20:61–73. https://doi.org/10.1006/snmr.2001.0030
Vieira MA, Gonçalves GR, Cipriano DF, Schettino MA, Silva Filho EA, Cunha AG, Emmerich FG, Freitas JCC (2016) Synthesis of graphite oxide from milled graphite studied by solid-state 13C nuclear magnetic resonance. Carbon 98:496–503. https://doi.org/10.1016/j.carbon.2015.11.037
Freitas JCC (2019) On the difficulties and pitfalls with the analysis of solid-state 13C NMR spectra in graphitic materials. Appl Magn Reson 50:1245–1252. https://doi.org/10.1007/s00723-019-01151-7
Mazur AS, Vovk MA, Tolstoy PM (2020) Solid-state 13C NMR of carbon nanostructures (milled graphite, graphene, carbon nanotubes, nanodiamonds, fullerenes) in 2000–2019: a mini-review. Fuller Nanotub Carbon Nanostructures 28:202–213
Bren KL (2011) Nuclear magnetic resonance (NMR) spectroscopy of metallobiomolecules. In: Encyclopedia of inorganic and bioinorganic chemistry https://doi.org/10.1002/9781119951438.eibc0296
Keating K, Knight R (2010) A laboratory study of the effect of Fe(II)-bearing minerals on nuclear magnetic resonance (NMR) relaxation measurements. Geophysics 75:F71–F82. https://doi.org/10.1190/1.3386573
Ẑujović Z, Srejić R, Vučelić D, Jovančićević B, Vitorović D (1998) Influence of depyritization on NMR relaxation parameters of Aleksinac oil shale kerogen. Fuel 77:1001–1003. https://doi.org/10.1016/S0016-2361(97)00282-2
Margenot AJ, Calderón FJ, Parikh SJ (2016) Limitations and potential of spectral subtractions in Fourier-transform infrared spectroscopy of soil samples. Soil Sci Soc Am J 80:10–26
Ţucureanu V, Matei A, Avram AM (2016) FTIR spectroscopy for carbon family study. Crit Rev Anal Chem 46:502–520. https://doi.org/10.1080/10408347.2016.1157013
Coates J (2006) Interpretation of infrared spectra, a practical approach. Encyclopedia of analytical chemistry: applications, theory and instrumentation https://doi.org/10.1002/9780470027318.a5606
Beyssac O, Goffé B, Chopin C, Rouzaud JN (2002) Raman spectra of carbonaceous material in metasediments: a new geothermometer. J Metamorph Geol 20:859–871. https://doi.org/10.1046/j.1525-1314.2002.00408.x
Huang E-P, Huang E, Yu S-C, Chen Y-H, Lee J-S, Fang J-N (2010) In situ Raman spectroscopy on kerogen at high temperatures and high pressures. Phys Chem Miner 37:593–600. https://doi.org/10.1007/s00269-010-0360-9
Heidarinejad Z, Dehghani MH, Heidari M, Javedan G, Ali I, Sillanpää M (2020) Methods for preparation and activation of activated carbon: a review. Environ Chem Lett 18:393–415. https://doi.org/10.1007/s10311-019-00955-0
Aylmore MG, de Klerk LW (2013) Conditions and design considerations for maximising recoverable gold in roasting of refractory gold ores. In: World gold 2013, Brisbane, Australia. AusIMM, Burwood, Victoria, Australia, pp. 375–387
Adams MD, Burger AM (1998) Characterization and blinding of carbonaceous preg-robbers in gold ores. Miner Eng 11:919–927. https://doi.org/10.1016/S0892-6875(98)00079-X
Santiago RCC, Ladeira ACQ (2019) Reduction of preg-robbing activity of carbonaceous gold ores with the utilization of surface blinding additives. Miner Eng 131:313–320. https://doi.org/10.1016/j.mineng.2018.11.029
Rees KL, van Deventer JSJ (2000) Preg-robbing phenomena in the cyanidation of sulphide gold ores. Hydrometallurgy 58:61–80. https://doi.org/10.1016/S0304-386X(00)00131-6
Agnico-Eagle Mines Limited (2011) The new gold standard. Paper presented at the Fennoscandian Exploration and Mining Conference, Levi, Lapland, Finland, 1–3 Nov 2011
Afenya PM (1991) Treatment of carbonaceous refractory gold ores. Miner Eng 4:1043–1055. https://doi.org/10.1016/0892-6875(91)90082-7
Guay WJ (1981) The treatment of refractory gold ores containing carbonaceous materials and sulphides. In: 110th AIME Meeting on Gold and Silver-leaching, Chicago, Illinois
Gallagher NP, Hendrix JL, Milosavljevic EB, Nelson JH, Solujic L (1990) Affinity of activated carbon towards some gold(I) complexes. Hydrometallurgy 25:305–316. https://doi.org/10.1016/0304-386X(90)90046-5
Aylmore MG (2016) Chapter 28 - thiosulfate as an alternative lixiviant to cyanide for gold ores. In: Adams MD (ed) Gold ore processing, 2nd edn. Elsevier, Amsterdam, pp 485–523. https://doi.org/10.1016/B978-0-444-63658-4.00028-1
Dai X, Bruer P, Hewitt d, Bergamin A (2013) Thiosulfate process for treating gold concentrates. In: World gold 2013, Brisbane, Australia. AusIMM, Burwood, Victoria, Australia, pp. 61–70
Acknowledgments
The authors would like to acknowledge the financial support provided by the ARC LP grant (LP160101760) for this work. We are thankful to Zijin Mining and the metallurgists and operators from the Shuiyindong gold mine for providing the ore samples associated with this work, and we greatly appreciate the support provided by the beamline scientists and staff of the Beijing Synchrotron Radiation Facility (BSRF). Special thanks to Nadia Zakhartchouk, Frank Antolasic, Stephen Grist, Bebeto Lay, Kyle Hearn, Zahra Homan, Peggy Chang, Sandro Longano, Cameron Crombie, Sanaz Salehi, Mary Karagiozakis, Mike Allan, the Rheology and Materials Processing Centre (RMPC), Babs Fairchild, Paul Jones, Dru Morrish, the Micro Nano Research Facility (MNRF), and the Centre for Advanced Materials and Industrial Chemistry (CAMIC) for training and the use of equipment. We would also like to thank Chris Sheedy, Winston Liew, Rong Fan and Jack Wrigley from CSIRO, as well as Lathe Jones, Nebeal Faris, Naresh Pillai, Selvakannan Perisamy, Yalong Ma and Yufang Zhang for their assistance at various stages of this study.
Funding
This study was funded by Zijin Mining and the Australian Research Council via the Linkage Project grant LP160101760.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of this study. Material preparation, data collection and analysis were performed by all authors at different stages of this study. The first draft of the manuscript was written by Wei Sung Ng and Yi Yang, and all authors have commented on the previous versions of the manuscript leading to this version. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no financial or non-financial interests in any material discussed in this article. At the time of writing, two co-authors, Xiuzhu Su, Shuiping Zhong, are employed by Zijin Mining.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 363 kb)
Rights and permissions
About this article
Cite this article
Ng, W.S., Yang, Y., Su, X. et al. Characterization of Preg-Robbing Carbonaceous Minerals from the Shuiyindong Carlin-Type Gold Deposit Via Spectroscopic Techniques. Mining, Metallurgy & Exploration 39, 169–188 (2022). https://doi.org/10.1007/s42461-021-00507-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00507-7