Abstract
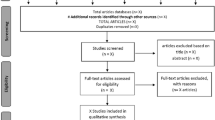
Pain is considered a public health problem due to its high economic cost, high prevalence, and great impact on the quality of life of patients and their families. Transcutaneous electrical nerve stimulation (TENS) has been shown to be a safe, inexpensive option with satisfactory results in improving pain control. However, the success of this therapy presents as key factors the parameters used, among them the intensity and duration of treatment. However, little is known about the validation of such equipment. To perform a systematic review of the equipment available for the application of electrostimulation as a therapeutic resource for analgesia, which had their methodological properties tested. A systematic literature search was conducted for all relevant scientific articles in the SCIELO, Medline/PubMed, PEDRo, and EBSCO databases. A total of 318 articles were found. Sixty-six were duplicate studies, 252 were selected by title, 72 by abstract, 5 by text full reading, and finally only 2 were included, since the others did not meet the pre-established inclusion criteria. The evidence gathered in this study shows that the performance evaluation of commercially available electrostimulation equipment may be a responsibility of the national regulatory agency. Our findings reinforce the importance of greater availability of information about the regulatory processes of these devices and the verification of the parameters of the regulated devices, so that the achievement of therapeutic goals is not compromised.



Similar content being viewed by others
Data Availability
The detailed strategy and the operational definitions of qualitative analysis are available in Appendix 1 and 2.
References
ABNT NBR ISO 5725. Accuracy (accuracy and precision) of measurement methods and results. Part 1: General Principles and Definitions. Rio de Janeiro, 2018.
ABNT NBR ISO 17665-1:2010. Sterilization of health products - steam. Brazilian Association of Technical Standards. ABNT NBR ISO 17665-1: 2010. Sterilization of health products - Steam. Brazilian Association of Technical Standards.
Brasil AN. Resolution-RDC No. 15 of March 15, 2012. Provides for best practice requirements for the processing of health products and other measures. Brasilia 2012
Brazilian Association of Technical Standards. NBR 5462: reliability and maintainability. ABNT; 1994
Chui QS, Barros CB, Silva TD. Repeatability (r) and reproducibility (R) indexes from interlaboratorial program: how to use them. Química Nova. 2009;32(8):2209–13.
Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018 Sep 14;67(36):1001–1006. https://doi.org/10.15585/mmwr.mm6736a2.
Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016 Jun 20;6(6):e010364. https://doi.org/10.1136/bmjopen-2015-010364.
Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PloS One. 2016;11(3):e0150205.
Garcia JB, Neto JO, Rodrigues TA. The role of academic leagues as a strategy for pain education in Brazil. J Pain Res. 2019;12:1891–8.
Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–24.
Gewandter JS, Chaudari J, Ibegbu C, Kitt R, Serventi J, Burke J, et al. Wireless transcutaneous electrical nerve stimulation device for chemotherapy-induced peripheral neuropathy: an open-label feasibility study. Support Care Cancer. 2019;27(5):1765–74.
Gibson W, Wand BM, Meads C, Catley MJ, O’connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2019;2019.
Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11(1):770.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928.
Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database Syst Rev, Issue. 2017:10. https://doi.org/10.1002/14651858.CD012172.pub2.
Jonas WB, Crawford C, Colloca L, Kriston L, Linde K, Moseley B, et al. Are invasive procedures effective for chronic pain? A systematic review. Pain Med. 2019;20(7):1281–93.
Klimas J, Gorfinkel L, Fairbairn N, Amato L, Ahamad K, Nolan S, et al. Strategies to identify patient risks of prescription opioid addiction when initiating opioids for pain: a systematic review. JAMA Netw Open. 2019;2(5):e193365.
Kolski MC, O’Connor A, Van Der Laan K, Lee J, Kozlowski AJ, Deutsch A. Validation of a pain mechanism classification system (PMCS) in physical therapy practice. J Manual Manip Ther. 2016;24(4):192–9.
Lecybyl R, Acosta J, Ghoshdastidar J, Stringfellow K, Hanna M. Validation, reproducibility and safety of trans dermal electrical stimulation in chronic pain patients and healthy volunteers. BMC Neurol. 2010;10(1):5.
Mills S, Torrance N, Smith BH. Identification and management of chronic pain in primary care: a review. Curr Psychiatry Rep. 2016;18(2):22.
Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019 Aug;123(2):e273–e283. https://doi.org/10.1016/j.bja.2019.03.023.
Morgan CR, Santos FS. Estudo da estimulação elétrica nervosa transcutânea (TENS) nível sensório para efeito de analgesia em pacientes com osteoartrose de joelho. Fisioterapia em Movimento, v. 24, n. 4, p. 637–646, 2011.
Ostojic K, Paget S, Kyriagis M, Morrow A. Acute and chronic pain in children and adolescents with cerebral palsy: prevalence, interference, and management. Arch Phys Med Rehab. 2019;12.
Pedott AH, Fogliatto FS. Repeatability and reproducibility studies of functional data. Production. 2013 Sep;23(3):548–60.
Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019 Feb;20(2):146–160. https://doi.org/10.1016/j.jpain.2018.07.006.
Portney, L. G., and M. Watkins. Foundations of clinical research: applications to practice: FA Davis Company. (2015).
Resende L, Meriwether E, Rampazo EP, Dailey D, Embree J, Deberg J, et al. Meta-analysis of transcutaneous electrical nerve stimulation for relief of spinal pain. Eur J Pain (London, England). 2018;22(4):663–78. https://doi.org/10.1102/ejp.1168.
Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7.
Van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111(1):13–8.
Acknowledgements
The authors would like to thank the library of FUMEC University, for its assistance in the systematic search of scientific articles.
Funding
This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
A statement specifying the contributions of every author should be added.
Igor Batista Guimarães: conceptualization, methodology, writing – review and editing, visualization, supervision. Mariana Ribeiro Volpini Lana: conceptualization, methodology, resources, formal analysis, investigation, writing – original draft, visualization, supervision. Mariana R C Aquino: methodology, software, formal analysis, investigation, writing – original draft, visualization. Jessé Mendonça Cavalheiro: conceptualization, methodology, visualization. Davi Neiva Alves: methodology, writing – review and editing, visualization. Claysson Bruno Santos Vimieiro: writing – review and editing, visualization, supervision.
Corresponding author
Ethics declarations
Ethics Approval
This study did not involve research with human beings, being a systematic review of scientific papers. Therefore, its submission was not necessary to the appreciation of an Ethics in Research Committee (CEP).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Appendices
Appendix 1. Detailed strategy for each source
English
EBSCO
Bireme
http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/
-
1.2.
SCIELO
Medline/PubMed
https://www.ncbi.nlm.nih.gov/pubmed/?term=Transcutaneous+Electric+Nerve+Stimulation
PEDro
Spanish
2.1. EBSCO
Bireme
http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/
SCIELO
Medline/PubMed
Portuguese
EBSCO
Bireme
http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/
SCIELO
Medline/PubMed
Appendix 2. Risk of bias criteria: operational definitions
Adequate sequence generation?
-
1.
Low risk of bias: based on a random component judged to be both appropriate and sufficiently well described.
-
2.
High risk of bias: based on any non-random component.
-
3.
Unclear risk of bias: insufficient information regarding sequence generation process to permit judgment of low or high risk of bias.
Adequate allocation concealment?
-
1.
Low risk of bias: method of concealment allocation employed prohibited foresight of participant assignment.
-
2.
High risk of bias: method of concealment allocation employed permitted possible foresight of participant assignment.
-
3.
Unclear risk of bias: method of concealment allocation not described or described in insufficient detail to permit judgment of low or high risk of bias.
Blinding of outcome assessment?
-
1.
Low risk of bias: outcome assessor (including participants with respect to self-reported outcomes) blinded to participants’ allocated intervention, and unlikely that blinding broken; OR no or incomplete blinding but judged that a given outcome unlikely to be influenced by lack of blinding.
-
2.
High risk of bias: outcome assessor (including participants with respect to self-report outcomes) unblinded to participants’ allocated intervention; OR outcome assessor blinded to allocated intervention but likely that blinding may have been broken (and a given outcome is likely to be influenced by lack of blinding).
-
3.
Unclear risk of bias: insufficient information to permit judgment of low or high risk of bias.
Blinding of participants?
-
1.
Low risk of bias: participants blinded to allocated intervention and unlikely that blinding broken; OR no or incomplete blinding but judged that a given outcome unlikely to be influenced by lack of blinding.
-
2.
High risk of bias: participants not blinded to allocated intervention; OR participants blinded to allocated intervention but likely that blinding may have been broken (and a given outcome is likely to be influenced by lack of blinding).
-
3.
Unclear risk of bias: insufficient information to permit judgment of low or high risk of bias.
Incomplete outcome data addressed?
-
1.
Low risk of bias: no missing outcome data. Reasons for missing data unlikely to be related to the true outcome. Missing outcome data balanced across intervention groups with similar reasons for omissions. Dichotomous outcomes: proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. Continuous outcomes: difference in means or standardized mean difference among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data imputed using appropriate methods. Intention-to-treat analysis undertaken. Less than or equal to 10% dropout rate.
-
2.
High risk of bias: reason for missing outcome data likely to be related to the true outcome. Dichotomous outcomes: proportion of missing outcomes compared with observed event risk enough to induce a clinically relevant bias in intervention effect estimate. Continuous outcomes: difference in means or standardized mean difference among missing outcomes enough to induce a clinically relevant bias on observed effect size. As-treated analysis undertaken with substantial departure of the intervention received from that assigned at randomization. Equal to or greater than 30% dropout rate.
-
3.
Unclear risk of bias: insufficient reporting of attrition or exclusions to permit judgment of low or high risk of bias. Greater than 10% and less than 30% dropout rate.
Selective outcome reporting?
-
1.
Low risk of bias: all primary outcomes of interest adequately reported with point estimates and measures of variance for all time points.
-
2.
High risk of bias: incomplete reporting of prespecified outcomes. One or more primary outcomes are reported using measurements, analysis methods, or subsets of data that were not prespecified. One or more reported primary outcomes were not prespecified. One or more outcomes of interest reported incompletely and cannot be entered into a meta-analysis. Results for a key outcome expected to have been reported, excluded.
-
3.
Unclear risk of bias: insufficient information to permit judgment of low or high risk of bias.
Free of other biases
-
1.
Low risk of bias: appears free of other sources of bias.
-
2.
High risk of bias: results may have been confounded by at least one important risk of bias (design-specific, fraudulent, other).
-
3.
Unclear risk of bias: other sources of bias may be present but there is either insufficient information to assess whether an important risk of bias exists; OR insufficient rationale or evidence regarding whether an identified problem will introduce bias.
Rights and permissions
About this article
Cite this article
Guimarães, I.B., Volpini Lana, M.R., de Aquino, M.R.C. et al. Methodological Properties of Transcutaneous Electrical Nerve Stimulation (TENS) Equipment Used for Analgesia in Humans: a Systematic Review. SN Compr. Clin. Med. 3, 1363–1372 (2021). https://doi.org/10.1007/s42399-021-00845-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00845-z