Abstract
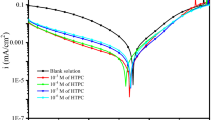
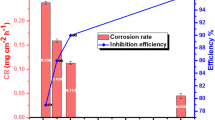
This work aims to study the inhibition ability of 2 Amino,4–6-imethylpyrimidine (ADMP) compound on the corrosion behavior of mild steel in 0.5 M sulfuric acid solution using electrochemical measurements and surface characterization. In addition, quantum chemical calculations were performed to investigate the inhibition mechanism. Monte Carlo simulations were utilized to determine the adsorption energy and configuration arrangement ADMP to the metal surface. The obtained results indicate that ADMP exhibits good inhibition performance for mild steel corrosion in acidic medium and acts as mixed-type inhibitor. The inhibition efficiency increases with the inhibitor concentration to reach 87%. Quantum chemical calculation and molecular dynamics (MD) simulation methods reveal that the inhibitor acts through the formation of a protective layer at the metal surface by the adsorption of its molecules onto mild steel surface. The adsorption is a mixed type of physical and chemical interactions and obeys Langmuir adsorption isotherm.












Similar content being viewed by others
References
Zhitao H, Junlin W, Yuqing W, Quanyou H, Shu-Feng L, Arumugam Z, Ramkumar S, Wu JJ, Asiri AM, Anandan S (2014) J Environ Chem Eng 2:463–470
Boutouil A, Laamari M, Elazhary I, Anane H, Ben Tama A, Stiriba S (2019) Anti-Corr Meth Mat 66:835–852
Jmiai A, El Ibrahimi B, Tara A, Chadili M, El Issami S, Jbara O, Khallaayoun A, Bazzi L (2018) J Molec Liq 268:102–113
Ouchenane S, Abderrahim K, Abderrahmane S, Bououdina M (2018) Mater Res Exp 5:106508
Bouklah M, Benchat N, Aouniti A, Hammouti B, Benkaddour M, Lagrenee M, Vezin H, Bentiss F (2004) Prog Org Coat 51:118–124
Wang L (2006) Corr Sci 48:608–616
Li W, Zhao X, Liu F, Hou B (2008) Corr Sci 50:3261–3266
Tao Z, Zhang S, Li W, Hou B (2009) Corr Sci 51:2588–2595
Obi-Egbedi NO, Obot IB (2013) Arab J Chem 6:211–223
Pavithra MK, Venkatesha TV, Vathsala K, Nayana KO (2010). Corr Sci. https://doi.org/10.1016/j.corsci.2010.07.034
Obot IB, Obi-Egbedi NO (2009) Corr Sci 52:276
Abd El Rehim SS, Sayyah SM, El-Deeb MM, Kamal SM, Azooz RE (2010) Mater Chem and Phys 123:20–27
Lenz DM, Delamar M, Ferreira CA (2003) J Electroanal Chem 54:35
El Azhar M, Traisnel M, Mernari B, Gengembre L, Bentiss F, Lagrenee M (2002) Appl Surf Sci 185:197
Dofe VS, Sarkate AP, Shaikh ZM, Jadhav CK, Nipte AS, Gill CH (2018) J Heterocyc Chem 55:756–762
Gregori T, Sedi M, Grb I, Tomljenovi PA, Kraljevi PS, Cetina M, Vianello R, Raimali S (2016) Eur J Med Chem 125:1247–1267
Verma C, Olasunkanmi LO, Ebenso EE, Quraishi MA, Obot IB (2016) J Phys Chem C 120:11598–11611
Li X, Xie X (2014) J Taiwan Inst Chem Eng 45:3033–3045
Li X, Deng S, Lin T, Xie X, Du G (2017) Corr Sci 118:202–216
Reznik V, Akamsin V, Khodyrev YP, Galiakberov R, Efremov YY, Tiwari L (2008) Corr Sci 50:392–403
Anusuya N, Saranya J, Sounthari P, Zarrouk A, Chitra S (2016). J Molec Liq. https://doi.org/10.1016/j.molliq.11.015
Hou BS, Zhang QH, Li YY, Zhu GY, Zhang GA (2020). Corr Sci. https://doi.org/10.1016/j.corsci.108442
Yadav M, Kumar S, Sinha RR, Bahadu I, Ebenso EE (2015) J Molec Liq 211:135–145
Haque J, Ansari KR, Srivastava V, Quraishi MA, Obot IB (2017) J Indus Engine Chem 49:176–188
Hou BS, Xu N, Zhang QH, Xuan CJ, Liu HF, Zhang GA (2018) J Taiwan Inst Chem Eng 95:541–554
Hejazi S, Mohajernia Sh, Moayed MH, Davoodi A, Rahimizadeh M, Momeni M, Eslami A, Shiri A, Kosari A (2015) J Indus Eng Chem 25:112–121
Xu Y, Zhang S, Li W, Guo L, Xu S, Feng L, Madkour LH (2018). Appl Surf Sci. https://doi.org/10.1016/j.apsusc.08.037
https://pubchem.ncbi.nlm.nih.gov/compound/2-Amino-4_6-dimethylpyrimidine.
Solmaz R, Sahin E, Döner A, Kardas G (2011) Corro Scie 53:3231–3240
Mu GN, Li XH, Qu Q, Zhou J (2006) Corr Sci 48:445–459
Farahati R, Ghaffarinejad A, Mousavi-Khoshdela SM, Rezania J, Behzadi H, Shockravi A (2019) Prog Org Coat J 132:417–428. https://doi.org/10.1016/j.porgcoat.2019.04.005
Bard AJ, Faulkner LR (2001) Electrochemical methods, fundamental and applications, 2nd edn. Wiley, New York
Crow DR (1979) Principles and applications of electrochemistry, 2nd edn. Nelson Thornes Ltd., London
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M, Hamadanian M, Gandomi A (2008) Corr Sci 50:2172–2181
Corrales-Luna M, Manh TL, Romero-Romo M, Palomar-Pardavé M, Arce-Estrada EM (2019) Corr Sci 153:85–99
Govindasamy R, Ayappan S (2015) J Chil Chem Soc 60:2786–2798
Kannan P, Karthikeyan J, Murugan P, Rao TS, Rajendran N (2016) J Mol Liq 221:368–380
Ansari KR, Quraishi MA (2014) J Ind Eng Chem 20:2819–2829
Tourabi M, Nohair K, Nyassi A, Hammouti B, Jama C, Bentiss F (2014) J Mater Environ Sci 4:1133–1143
Chraka A, Raissouni I, Benseddik N, Khayar S, Ibn Mansour A, Belcadi H, Chaouket F, Bouchta D (2020) Mater Today Proc 22:83–88
Orazem ME, Tribollet B (2008) Electrochemical impedance spectroscopy. Wiley, New Jersey
El-Kacimi Y, Touir R, Alaoui K, Kaya S, Salem Abousalem A, Ouakki M, Ebn Touhami M (2020) J Bio Tribo Corr 6:47
Akinbulumo OA, Odejobi OJ, Odekanle EL (2020) Results Mater 5:100074
Loto RT, Loto CA (2018) Results Phys 10:99–106
Amin MA (2006) J Appl Electrochem 36:215–226
El Azzouzi M, Aouniti A, Tighadouin S, Elmsellem H, Radi S, Hammouti B, El Assyry A, Bentiss F, Zarrouk A (2016) J Mol Liq 221:633–641
Rakashaiah P, Kumara BG, Pandith D, Shetty A, Amitha BE (2018). Corr Sci. https://doi.org/10.1016/j.corsci.03.021
Ankush AM, Chandrabhan V, Vandana S, Hassane L, Quraishi M, Ebenso E, Chung I (2018). J Bio Tribo Corr. https://doi.org/10.1007/s40735-018-0147-y
Dan W, Bin X, Yuanpeng L, Shan S, Chao L (2014) Corr Sci 85:77–86
Lebrini M, Lagrenee M, Traisnel M, Gengembre L, Vezin H, Bentiss F (2007) App Surf Sci 253:9267–9276
Khaled K (2009) Electrochem Acta 54:4345–4352
Nnabuk E, Eno E, Udo I (2009) J Argentine Chem Soc 97:178–194
Ahmed M, Mohamad AB, Mohd T, Ramzi J (2012). Res Chem Intermediates. https://doi.org/10.1007/s11164-011-0362-3
Blajiev O, Annick H (2004) Electrochem Acta 49:2761
Greenwood H (1952). J Chem Phys. https://doi.org/10.1063/1.1700243
Elayyachy M, Abderahmane EI, Hammouti B (2006) Corro Sci 48:2470–2479
Vandana S, Yadav M (2019) J Mol Liq 297:111883. https://doi.org/10.1016/j.molliq.2019.111883
Khaled K, Mohammed A, Al-Mobarak NA (2010) J Appl Electrochem 40:601–613
Wang CT, Chen SH, Ma HY, Qi CS (2003) J Appl Electrochem 33:179–186
Özcan G, İlyas D, Erbil M (2004) App Surf Sci 236:155–164
Hassane L, Fadoua E, Belghiti ME, Hammouti B, Shehdeh J, Othman H, Rachid S (2018). Portugaliae Electrochem Acta. https://doi.org/10.4152/pea.201803197
Vandana S, Yadav M (2019). J Mol Liq. https://doi.org/10.1016/j.molliq.2019.111883
Dagdag O, El Harfi A, El Gouri M, Safi Z, Jalgham RT, Wazzan N, Verma C, Ebenso EE, Kumar UP (2019). Heliyon. https://doi.org/10.1016/j.heliyon.2019.e01340
Hualiang H, Furong B (2019). Corr Sci. https://doi.org/10.1016/j.corsci.2019.108413
Acknowledgements
The authors are greatly thankful to Mining and Metallurgy National High School (ENSMM), Annaba, Algeria for SEM/EDS characterization measurements.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
SO and HS conceptualization, SR software, SO and MB validation, RJ data curation and theoretical study, SO writing—original draft preparation, MB visualization and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ouchenane, S., Jalgham, R.T.T., Rezgoun, S. et al. Experimental and Theoretical Studies of the Corrosion Inhibition Properties of 2 Amino, 4–6-Dimethylpyrimidine for Mild Steel in 0.5 M H2SO4. Chemistry Africa 4, 621–633 (2021). https://doi.org/10.1007/s42250-021-00239-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00239-7