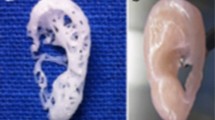
Abstract
At present, the clinical reconstruction of the auricle usually adopts the strategy of taking autologous costal cartilage. This method has great trauma to patients, poor plasticity and inaccurate shaping. Three-dimensional (3D) printing technology has made a great breakthrough in the clinical application of orthopedic implants. This study explored the combination of 3D printing and tissue engineering to precisely reconstruct the auricle. First, a polylactic acid (PLA) polymer scaffold with a precisely customized patient appearance was fabricated, and then auricle cartilage fragments were loaded into the 3D-printed porous PLA scaffold to promote auricle reconstruction. In vitro, gelatin methacrylamide (GelMA) hydrogels loaded with different sizes of rabbit ear cartilage fragments were studied to assess the regenerative activity of various autologous cartilage fragments. In vivo, rat ear cartilage fragments were placed in an accurately designed porous PLA polymer ear scaffold to promote auricle reconstruction. The results indicated that the chondrocytes in the cartilage fragments could maintain the morphological phenotype in vitro. After three months of implantation observation, it was conducive to promoting the subsequent regeneration of cartilage in vivo. The autologous cartilage fragments combined with 3D printing technology show promising potential in auricle reconstruction.
Graphic abstract










Similar content being viewed by others
References
Kim HY, Jung SY, Lee SJ et al (2019) Fabrication and characterization of 3D-printed elastic auricular scaffolds: a pilot study. Laryngoscope 129(2):351–357. https://doi.org/10.1002/lary.27344
Fu YY, Li CL, Zhang JL et al (2019) Autologous cartilage microtia reconstruction: complications and risk factors. Int J Pediatr Otorhinolaryngol 116:1–6. https://doi.org/10.1016/j.ijporl.2018.09.035
Jovic TH, Stewart K, Kon M et al (2020) Auricular reconstruction: a sociocultural, surgical and scientific perspective. J Plast Reconstr Aesthet Surg 73(8):1424–1433. https://doi.org/10.1016/j.bjps.2020.03.025
Arshad M, Shirani G, Refoua S (2018) Rehabilitation of an auricular defect using surgical stent. World J Plast Surg 7(1):113–117
Han SE, Lim SY, Pyon JK et al (2015) Aesthetic auricular reconstruction with autologous rib cartilage grafts in adult microtia patients. J Plast Reconstr Aesthet Surg 68(8):1085–1094. https://doi.org/10.1016/j.bjps.2015.04.016
Bly RA, Bhrany AD, Murakami CS et al (2016) Microtia reconstruction. Facial Plast Surg Clin North Am 24(4):577–591. https://doi.org/10.1016/j.fsc.2016.06.011
Siegert R, Magritz R (2019) Otoplasty and auricular reconstruction. Facial Plast Surg 35(4):377–386. https://doi.org/10.1055/s-0039-1693745
Cenzi R, Farina A, Zuccarino L et al (2005) Clinical outcome of 285 medpor grafts used for craniofacial reconstruction. J Craniofac Surg 16(4):526–530. https://doi.org/10.1097/01.scs.0000168761.46700.dc
Jang CH, Koo Y, Kim G (2020) ASC/chondrocyte-laden alginate hydrogel/PCL hybrid scaffold fabricated using 3D printing for auricle regeneration. Carbohydr Polym 248:116776. https://doi.org/10.1016/j.carbpol.2020.116776
Uto S, Hikita A, Sakamoto T et al (2021) Ear cartilage reconstruction combining induced pluripotent stem cell-derived cartilage and three-dimensional shape-memory scaffold. Tissue Eng A 27(9–10):604–617. https://doi.org/10.1089/ten.TEA.2020.0106
Yue H, Pathak JL, Zou R et al (2021) Fabrication of chondrocytes/chondrocyte-microtissues laden fibrin gel auricular scaffold for microtia reconstruction. J Biomater Appl 35(7):838–848. https://doi.org/10.1177/0885328220954415
Hutmacher DW (2000) Scaffolds in tissue engineering bone and cartilage. Biomaterials 21(24):2529–2543. https://doi.org/10.1016/s0142-9612(00)00121-6
Langer R, Vacanti JP (1993) Tissue engineering. Science 260(5110):920–926. https://doi.org/10.1126/science.8493529
Kim S, Lee M (2020) Rational design of hydrogels to enhance osteogenic potential. Chem Mater 32(22):9508–9530. https://doi.org/10.1021/acs.chemmater.0c03018
Armiento AR, Alini M, Stoddart MJ (2019) Articular fibrocartilage—why does hyaline cartilage fail to repair? Adv Drug Deliv Rev 146:289–305. https://doi.org/10.1016/j.addr.2018.12.015
Kwak ES (2010) Asian cosmetic facial surgery. Facial Plast Surg 26(2):102–109. https://doi.org/10.1055/s-0030-1253497
Min SH, Kim JH, Lee MI et al (2020) Evaluation of auricular cartilage reconstruction using a 3-dimensional printed biodegradable scaffold and autogenous minced auricular cartilage. Ann Plast Surg 85(2):185–193. https://doi.org/10.1097/SAP.0000000000002313
Lee MR, Unger JG, Rohrich RJ (2011) Management of the nasal dorsum in rhinoplasty: a systematic review of the literature regarding technique, outcomes, and complications. Plast Reconstr Surg 128(5):538e–550e. https://doi.org/10.1097/PRS.0b013e31822b6a82
Gane S, East C, Jayaraj S et al (2007) Rolled auricular cartilage grafts for dorsal augmentation rhinoplasty. J Laryngol Otol 121(4):387–389. https://doi.org/10.1017/S0022215106003483
Lin AJ, Bernstein JL, Spector JA (2018) Ear reconstruction and 3D printing: is it reality? Current Surg Rep 6(2):4. https://doi.org/10.1007/s40137-018-0198-5
Song P, Hu C, Pei X et al (2019) Dual modulation of crystallinity and macro-/microstructures of 3D printed porous titanium implants to enhance stability and osseointegration. J Mater Chem B 7(17):2865–2877. https://doi.org/10.1039/c9tb00093c
Ma H, Feng C, Chang J et al (2018) 3D-printed bioceramic scaffolds: from bone tissue engineering to tumor therapy. Acta Biomater 79:37–59. https://doi.org/10.1016/j.actbio.2018.08.026
Long J, Zhang W, Chen Y et al (2021) Multifunctional magnesium incorporated scaffolds by 3D-printing for comprehensive postsurgical management of osteosarcoma. Biomaterials 275:120950. https://doi.org/10.1016/j.biomaterials.2021.120950
Zhu YZ, Joralmon D, Shan WT et al (2021) 3D printing biomimetic materials and structures for biomedical applications. Bio-Des Manuf 4(2):405–428. https://doi.org/10.1007/s42242-020-00117-0
Lee JS, Hong JM, Jung JW et al (2014) 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 6(2):024103. https://doi.org/10.1088/1758-5082/6/2/024103
Yin ZQ, Li D, Liu Y et al (2020) Regeneration of elastic cartilage with accurate human-ear shape based on PCL strengthened biodegradable scaffold and expanded microtia chondrocytes. Appl Mater Today 20:100724. https://doi.org/10.1016/j.apmt.2020.100724
Mukherjee P, Chung J, Cheng K et al (2021) Invitro and invivo study of PCL-hydrogel scaffold to advance bioprinting translation in microtia reconstruction. J Craniofac Surg 32(5):1931–1936. https://doi.org/10.1097/SCS.0000000000007173
Milazzo M, Negrini NC, Scialla S et al (2019) Additive manufacturing approaches for hydroxyapatite-reinforced composites. Adv Funct Mater 29(35):1903055. https://doi.org/10.1002/adfm.201903055
Wu L, Gu Y, Liu L et al (2020) Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials 227:119555. https://doi.org/10.1016/j.biomaterials.2019.119555
Montesdeoca CYC, Afewerki S, Stocco TD et al (2020) Oxygen-generating smart hydrogels supporting chondrocytes survival in oxygen-free environments. Colloids Surf B Biointerfaces 194:111192. https://doi.org/10.1016/j.colsurfb.2020.111192
De Moor L, Minne M, Tytgat L et al (2021) Tuning the phenotype of cartilage tissue mimics by varying spheroid maturation and methacrylamide-modified gelatin hydrogel characteristics. Macromol Biosci 21(5):e2000401. https://doi.org/10.1002/mabi.202000401
Ruiz-Cantu L, Gleadall A, Faris C et al (2020) Multi-material 3D bioprinting of porous constructs for cartilage regeneration. Mater Sci Eng C Mater Biol Appl 109:110578. https://doi.org/10.1016/j.msec.2019.110578
Arora A, Kothari A, Katti DS (2016) Pericellular plasma clot negates the influence of scaffold stiffness on chondrogenic differentiation. Acta Biomater 46:68–78. https://doi.org/10.1016/j.actbio.2016.09.038
Kapr J, Petersilie L, Distler T et al (2021) Human induced pluripotent stem cell-derived neural progenitor cells produce distinct neural 3D in vitro models depending on alginate/gellan gum/laminin hydrogel blend properties. Adv Healthc Mater 10(16):e2100131. https://doi.org/10.1002/adhm.202100131
Zhang BQ, Wang L, Song P et al (2021) 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des 201:109490. https://doi.org/10.1016/j.matdes.2021.109490
Wang C, Feng N, Chang F et al (2019) Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv Healthc Mater 8(14):e1900312. https://doi.org/10.1002/adhm.201900312
Ishikawa S, Iijima K, Matsukuma D et al (2020) Enhanced function of chondrocytes in a chitosan-based hydrogel to regenerate cartilage tissues by accelerating degradability of the hydrogel via a hydrolysable crosslinker. J Appl Polym Sci 137(19):48893. https://doi.org/10.1002/app.48893
Salzmann GM, Ossendorff R, Gilat R et al (2021) Autologous minced cartilage implantation for treatment of chondral and osteochondral lesions in the knee joint: an overview. Cartilage 13(1_suppl):1124S–1136S. https://doi.org/10.1177/1947603520942952
Kim IL, Mauck RL, Burdick JA (2011) Hydrogel design for cartilage tissue engineering: a case study with hyaluronic acid. Biomaterials 32(34):8771–8782. https://doi.org/10.1016/j.biomaterials.2011.08.073
Huey DJ, Hu JC, Athanasiou KA (2012) Unlike bone, cartilage regeneration remains elusive. Science 338(6109):917–921. https://doi.org/10.1126/science.1222454
Van Gastel N, Stegen S, Eelen G et al (2020) Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature 579(7797):111–117. https://doi.org/10.1038/s41586-020-2050-1
Bonasia DE, Marmotti A, Mattia S et al (2015) The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: an in vitro study. Arthroscopy 31(12):2335–2341. https://doi.org/10.1016/j.arthro.2015.06.025
Lu Y, Dhanaraj S, Wang Z et al (2006) Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res 24(6):1261–1270. https://doi.org/10.1002/jor.20135
Bugbee W, Cavallo M, Giannini S (2012) Osteochondral allograft transplantation in the knee. J Knee Surg 25(2):109–116. https://doi.org/10.1055/s-0032-1313743
Zingler C, Carl HD, Swoboda B et al (2016) Limited evidence of chondrocyte outgrowth from adult human articular cartilage. Osteoarthritis Cartilage 24(1):124–128. https://doi.org/10.1016/j.joca.2015.07.014
Tsuyuguchi Y, Nakasa T, Ishikawa M et al (2021) The benefit of minced cartilage over isolated chondrocytes in atelocollagen gel on chondrocyte proliferation and migration. Cartilage 12(1):93–101. https://doi.org/10.1177/1947603518805205
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81171731), the Project of Chengdu Science and Technology Bureau (Nos. 2021-YF05-01619-SN and 2021-RC05-00022-CG), the Science and Technology Project of Tibet Autonomous Region (Nos. XZ202202YD0013C and XZ201901-GB-08), the Sichuan Science and Technology Program (No. 2022YFG0066), and the 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Nos. ZYJC21026, ZYGD21001 and ZYJC21077).
Author information
Authors and Affiliations
Contributions
CCZ, YJF and XDZ conceived and designed the experiments. XYG, PS and ZYP performed the experiments. LC and XJX prepared the figures. HRL and PT consulted the relevant literature. XYG, YXW and ZXS analyzed the data. QQK and ZYZ provided guidance on cell and animal experiments. XYG, PS and CCZ wrote the paper. YJF, ZYL and YC made revisions to the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were approved by the Animal Care and Use Committee of Sichuan University (KS2019022-2).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gui, X., Peng, Z., Song, P. et al. 3D printing of personalized polylactic acid scaffold laden with GelMA/autologous auricle cartilage to promote ear reconstruction. Bio-des. Manuf. 6, 451–463 (2023). https://doi.org/10.1007/s42242-023-00242-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42242-023-00242-6