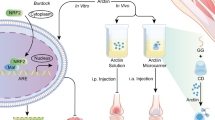
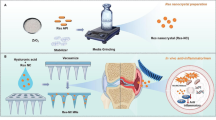
Abstract
Sports osteoarthritis (OA) is the most prevalent chronic joint disease in professional and recreational athletes. Resveratrol (RES), which is extracted from plants, has excellent anti-inflammatory properties. However, its application in OA treatment is limited by its poor water solubility, low bioavailability, and rapid metabolism. In this study, a silica aerogel (SA) was prepared through the sol–gel method and used to carry RES in the form of a nanodrug complex system (RSA) with a high drug–loading rate of 21.8% and a sustained release effect that lasted more than 6 hours. RSA had good biocompatibility in human chondrocytes when the concentration was less than 40 μg/mL and enhanced the anti-inflammatory actions of RES in vitro. A rat model of exercise-induced osteoarthritis was constructed to examine the therapeutic effects of RSA in vivo. Furthermore, the inflammatory factors involved in cartilage degradation and catabolism were reduced to 14.6–19.1% those of the original values after RES intervention, while the expression of type II collagen significantly increased. Moreover, RES was found to activate SIRT-1 by inhibiting the NF-κB-mediated inflammatory pathway, which also alleviated the degradation of cartilage matrix. These results indicated that the use of RSA could provide a novel noninvasive approach for targeted OA therapy with the potential for oral drug delivery.
Graphical abstract

A resveratrol-silica aerogel (RSA) nanodrug complex system with a high drug–loading rate and a sustained release effect efficiently enhance the treatment of sports osteoarthritis by activating SIRT-1 through oral administration.






Similar content being viewed by others
Data availability
Some or all data or models used during the study are available from the corresponding author by request.
References
Litwic A, Edwards MH, Dennison EM, Cooper C (2013) Epidemiology and burden of osteoarthritis. Br Med Bull 105(1):185–199. https://doi.org/10.1093/bmb/lds038
Malfait AM (2016) Osteoarthritis year in review 2015: biology. Osteoarthritis Cartilage 24(1):21–26. https://doi.org/10.1016/j.joca.2015.09.010
Wang KZ, Lei GH, Hu YC (2021) Chinese guideline for diagnosis and treatment of osteoarthritis. Chinese J Orthop 41(18):1291–1314. https://doi.org/10.3760/cma.j.cn121113-20210624-00424
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE (2019) OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil 27(11):1578–1589. https://doi.org/10.1016/j.joca.2019.06.011
Persson MSM, Stocks J, Varadi G, Hashempur MH, Van Middelkoop M, Bierma-Zeinstra S, Walsh DA, Doherty M, Zhang W (2020) Predicting response to topical non-steroidal anti-inflammatory drugs in osteoarthritis: an individual patient data meta-analysis of randomized controlled trials. Rheumatol 59(9):2207–2216. https://doi.org/10.1093/rheumatology/keaa113
Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, van Wijnen AJ, Im HJ (2013) A current review of molecular mechanisms regarding osteoarthritis and pain. Gene 527(2):440–447. https://doi.org/10.1016/j.gene.2013.05.069
Kang DG, Lee HJ, Lee CJ, Park JS (2018) Inhibition of the expression of matrix metalloproteinases in articular chondrocytes by resveratrol through affecting nuclear factor-kappa b signaling pathway. Biomol Ther 26(6):560–567. https://doi.org/10.4062/biomolther.2018.132
Collins JA, Moots RJ, Clegg PD, Milner PI (2015) Resveratrol and N-acetylcysteine influence redox balance in equine articular chondrocytes under acidic and very low oxygen conditions. Free Radic Biol Med 86:57–64. https://doi.org/10.1016/j.freeradbiomed.2015.05.008
Wenzel E, Somoza V (2005) Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res 49(5):472–481. https://doi.org/10.1002/mnfr.200500010
Montsko G, Nikfardjam MSP, Szabo Z, Boddi K, Lorand T, Ohmacht R, Mark L (2008) Determination of products derived from trans-resveratrol UV photoisomerisation by means of HPLC-APCI-MS. J Photochem Photobiol A Chem 196(1):44–50. https://doi.org/10.1016/j.jphotochem.2007.11.011
Wang W, Sun L, Zhang P, Song J, Liu W (2014) An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater 10(12):4983–4995. https://doi.org/10.1016/j.actbio.2014.08.022
Choi SM, Lee KM, Ryu SB, Park YJ, Hwang YG, BaekD CY, Park KH, Park KD, Lee JW (2018) Enhanced articular cartilage regeneration with SIRT1-activated MSCs using gelatin-based hydrogel. Cell Death Dis 9(9):886. https://doi.org/10.1038/s41419-018-0914-1
Sheu SY, Chen WS, Sun JS, Lin FH, Wu T (2013) Biological characterization of oxidized hyaluronic acid/resveratrol hydrogel for cartilage tissue engineering. J Biomed Mater Res - Part A 101(12):3457–3466. https://doi.org/10.1002/jbm.a.34653
Wu G, Wang L, Li H, Ke Y, Yao Y (2016) Function of sustained released resveratrol on IL-1β-induced hBMSC MMP13 secretion inhibition and chondrogenic differentiation promotion. J Biomater Appl 30(7):930–939. https://doi.org/10.1177/0885328215614425
Kann B, Spengler C, Coradini K, Rigo LA, Bennink ML, Jacobs K, Offerhaus HL, Beck RCR, Windbergs M (2016) Intracellular delivery of poorly soluble polyphenols: elucidating the interplay of self-assembling nanocarriers and human chondrocytes. Anal Chem 88(14):7014–7022. https://doi.org/10.1021/acs.analchem.6b00199
Le Clanche S, Cheminel T, Rannou F, Bonnefont-Rousselot D, Borderie D, Charrueau C (2018) Use of resveratrol self-emulsifying systems in T/C28a2 cell line as beneficial effectors in cellular uptake and protection against oxidative stress-mediated death. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00538
Wang J, Wang HY, Zhu RR, Liu Q, Fei J, Wang SL (2015) Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in IL-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 53:475–483. https://doi.org/10.1016/j.biomaterials.2015.02.116
Yang L, He XL, Jing GX, Wang H, Niu JH, Qian YC, Wang SL (2021) Layered double hydroxide nanoparticles with osteogenic effects as miRNA carriers to synergistically promote osteogenesis of MSCs. ACS Appl Mater Interfaces 13(41):48386–48402. https://doi.org/10.1021/acsami.1c14382
Zhu RR, Zhu XF, Zhu YJ, Wang ZJ, He XL, Wu ZR, Xue L, Fan WY, Huang RQ, Xu Z, Qi X, Xu W, Yu Y, Ren YL, Li C, Cheng Q, Ling L, Wang SL, Cheng LM (2021) Immunomodulatory layered double hydroxide nanoparticles enable neurogenesis by targeting transforming growth factor-β receptor 2. ACS Nano 15(2):2812–2830. https://doi.org/10.1021/acsnano.0c08727
Ji XJ, Zhong Y, Li CY, Chu JJ, Wang HQ, Xing Z, Niu TT, Zhang ZH, Du A (2021) Nanoporous carbon aerogels for laser-printed wearable sensors. ACS Appl Nano Mater 4(7):6796–6804. https://doi.org/10.1021/acsanm.1c00858
Ji XJ, Wang HQ, Chen TZ, Zhang T, Chu JJ, Du A (2020) Intrinsic negative TCR of superblack carbon aerogel films and their ultrabroad band response from UV to microwave. Carbon 161:590–598. https://doi.org/10.1016/j.carbon.2020.01.101
Yang JM, Cui NX, Han DX, Shen J, Wu GM, Zhang ZH, Qin LL, Zhou B, Du A (2021) A simple strategy for constructing hierarchical composite electrodes of PPy-posttreated 3D-printed carbon aerogel with ultrahigh areal capacitance over 8000 mF cm-2. Adv Mater Technol 2101325. https://doi.org/10.1002/admt.202101325
Riley L, Schirmer L, Segura T (2019) Granular hydrogels: emergent properties of jammed hydrogel microparticles and their applications in tissue repair and regeneration. Curr Opin Biotechnol 60:1–8. https://doi.org/10.1016/j.copbio.2018.11.001
Xie PT, Sun W, Du A, Hou Q, Wu GM, Fan RH (2021) Epsilon-negative carbon aerogels with state transition from dielectric to degenerate semiconductor. Adv Electron Mater 7(3). https://doi.org/10.1002/aelm.202000877
Sun W, Du A, Feng Y, Shen J, Huang SM, Tang J, Zhou B (2016) Super black material from low-density carbon aerogels with subwavelength structures. ACS Nano 10(10):9123–9128. https://doi.org/10.1021/acsnano.6b02039
Xie PT, Sun W, Liu Y, Du A, Zhang ZD, Wu GM, Fan RH (2018) Carbon aerogels towards new candidates for double negative metamaterials of low density. Carbon 129:598–606. https://doi.org/10.1016/j.carbon.2017.12.009
Wu S, Du A, Huang SM, Sun W, Zu GQ, Xiang YL, Li CH, Zhou B (2016) Effects of monomer rigidity on the microstructures and properties of polyimide aerogels cross-linked with low cost aminosilane. RSC Adv 6(27):22868–22877. https://doi.org/10.1039/C5RA28152K
Du A, Zhou B, Zhang ZH, Shen J (2013) A special material or a new state of matter: a review and reconsideration of the aerogel. Materials 6(3):941–968. https://doi.org/10.3390/ma6030941
Stergar J, Maver U (2016) Review of aerogel-based materials in biomedical applications. J Sol-Gel Sci Technol 77(3):738–752. https://doi.org/10.1007/s10971-016-3968-5
Liu MF, Du A, Li TM, Zhang T, Zhang ZH, Cao GW, Li HW, Shen J, Zhou B (2019) Simple deceleration mechanism confirmed in the terminal hypervelocity impacted tracks in SiO2 aerogel. Icarus 317:365–372. https://doi.org/10.1016/j.icarus.2018.08.017
Du A, Ma Y, Liu MF, Zhang ZH, Cao GW, Li HW, Wang L, Si PJ, Shen J, Zhou B (2021) Morphology analysis of tracks in the aerogels impacted by hypervelocity irregular particles. High Power Laser Sci and Eng 9(2):e14. https://doi.org/10.1017/hpl.2020.54
Alemán J, Chadwick AV, He J, Hess M, Horie K, Jones RG, Kratochvíl P, Meisel I, Mita I, Moad G, Penczek S, Stepto RFT (2007) Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl Chem 79(10):1801–1827. https://doi.org/10.1351/pac200779101801
Juère E, Florek J, Bouchoucha M, Jambhrunkar S, Wong KY, Popat A, Kleitz F (2017) In vitro dissolution, cellular membrane permeability, and anti-inflammatory response of resveratrol-encapsulated mesoporous silica nanoparticles. Mol Pharm 14(12):4431–4441. https://doi.org/10.1021/acs.molpharmaceut.7b00529
Summerlin N, Qu Z, Pujara N, Sheng Y, Jambhrunkar S, McGuckin M, Popat A (2016) Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloid Surf B-Biointerfaces 144:1–7. https://doi.org/10.1016/j.colsurfb.2016.03.076
Qin LL, He YW, Zhao XY, Zhang T, Qin Y, Du A (2020) Preparation, characterization, and in vitro sustained release profile of resveratrol-loaded silica aerogel. Molecules 25(12):2752. https://doi.org/10.3390/molecules25122752
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7(1):33–42. https://doi.org/10.1038/nrrheum.2010.196
Alaaeddine N, DiBattista JA, Pelletier JP, Cloutier JM, Kiansa K, Dupuis M, Martel-Pelletier J (1997) Osteoarthritic synovial fibroblasts possess an increased level of tumor necrosis factor-receptor 55 (TNF-R55) that mediates biological activation by TNF-alpha. J Rheumatol 24(10):1985–1994
Naume B, Shalaby R, Lesslauer W, Espevik T (1991) Involvement of the 55- and 75-kDa tumor necrosis factor receptors in the generation of lymphokine-activated killer cell activity and proliferation of natural killer cells. J Immunol 146(9):3045–3048
Guerne PA, Carson DA, Lotz M (1990) IL-6 production by human articular chondrocytes-modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 144(2):499–505
Bender S, Haubeck HD, Van de Leur E, Dufhues G, Schiel X, Lauwerijns J, Greiling H, Heinrich PC (1990) Interleukin-1β induces synthesis and secretion of interleukin-6 in human chondrocytes. FEBS Lett 263(2):321–324. https://doi.org/10.1016/0014-5793(90)81404-C
Setton LA, Elliott DM, Mow VC (1999) Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage 7(1):2–14. https://doi.org/10.1053/joca.1998.0170
Mäkelä JTA, Han SK, Herzog W, Korhonen RK (2015) Very early osteoarthritis changes sensitively fluid flow properties of articular cartilage. J Biomech 48(12):3369–3376. https://doi.org/10.1016/j.jbiomech.2015.06.010
Li YS, Xiao WF, Wu P, Deng ZH, Zeng C, Li H, Yang TH, Lei G (2016) The expression of SIRT1 in articular cartilage of patients with knee osteoarthritis and its correlat. J Orthop Surg Res 11(1):144. https://doi.org/10.1186/s13018-016-0477-8
Yamamoto T, Miyaji N, Kataoka K, Nishida K, Nagai K, Kanzaki N, HoshinoY KR, Matsushita T (2021) Knee osteoarthritis progression is delayed in silent information regulator 2 ortholog 1 knock-in Mice. Int J Mol Sci 22(19):10689. https://doi.org/10.3390/ijms221910685
Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cel Signal 25(10):1939–1948. https://doi.org/10.1016/j.cellsig.2013.06.007
Van den Berg WB (2011) Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthr Cartil 19(4):338–341. https://doi.org/10.1016/j.joca.2011.01.022
Matsushita T, Sasaki H, Takayama K, Ishida K, Matsumoto T, Kubo S, Matsuzaki T, Nishida K, Kurosaka M, Kuroda R (2013) The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1β in human chondrocytes. J Orthop Res 31(4):531–537. https://doi.org/10.1002/jor.22268
Funding
This work was funded by the National Key Research and Development Program of China (2017YFA0204600), the National Natural Science Foundation of China (Grant No. 31771313), and the China Postdoctoral Science Foundation (2021M692442).
Author information
Authors and Affiliations
Contributions
L.Q., A.D, and S.W. provided the conception and design of the study. L.Q., N.C., and Y.H. completed the material synthesis and animal experiments. L.Q. and G.J. completed the in vitro experiments. L.Q., A.D., G.J., and N.C. co-wrote the main manuscript text. Z.X., Y.Q., T.L., and J.S. performed image and data analyses. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, L., Jing, G., Cui, N. et al. Resveratrol-silica aerogel nanodrug complex system enhances the treatment of sports osteoarthritis by activating SIRT-1. Adv Compos Hybrid Mater 6, 3 (2023). https://doi.org/10.1007/s42114-022-00576-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42114-022-00576-2